Morphological Study of Insect Mechanoreceptors to Develop Artificial Bio-Inspired Mechanosensors †
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects
2.2. Preparation of Samples
2.3. Samples under SEM and TEM
3. Results
3.1. Sensilla on Antennal Segments External MORPHOLOGY
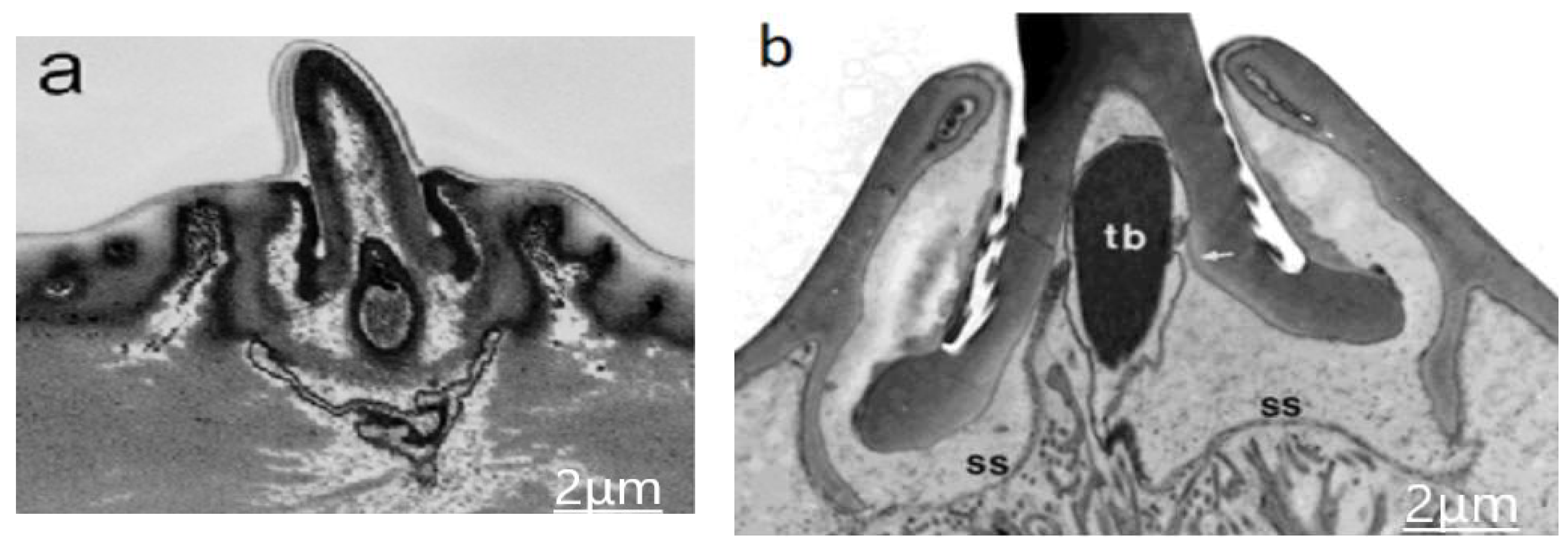
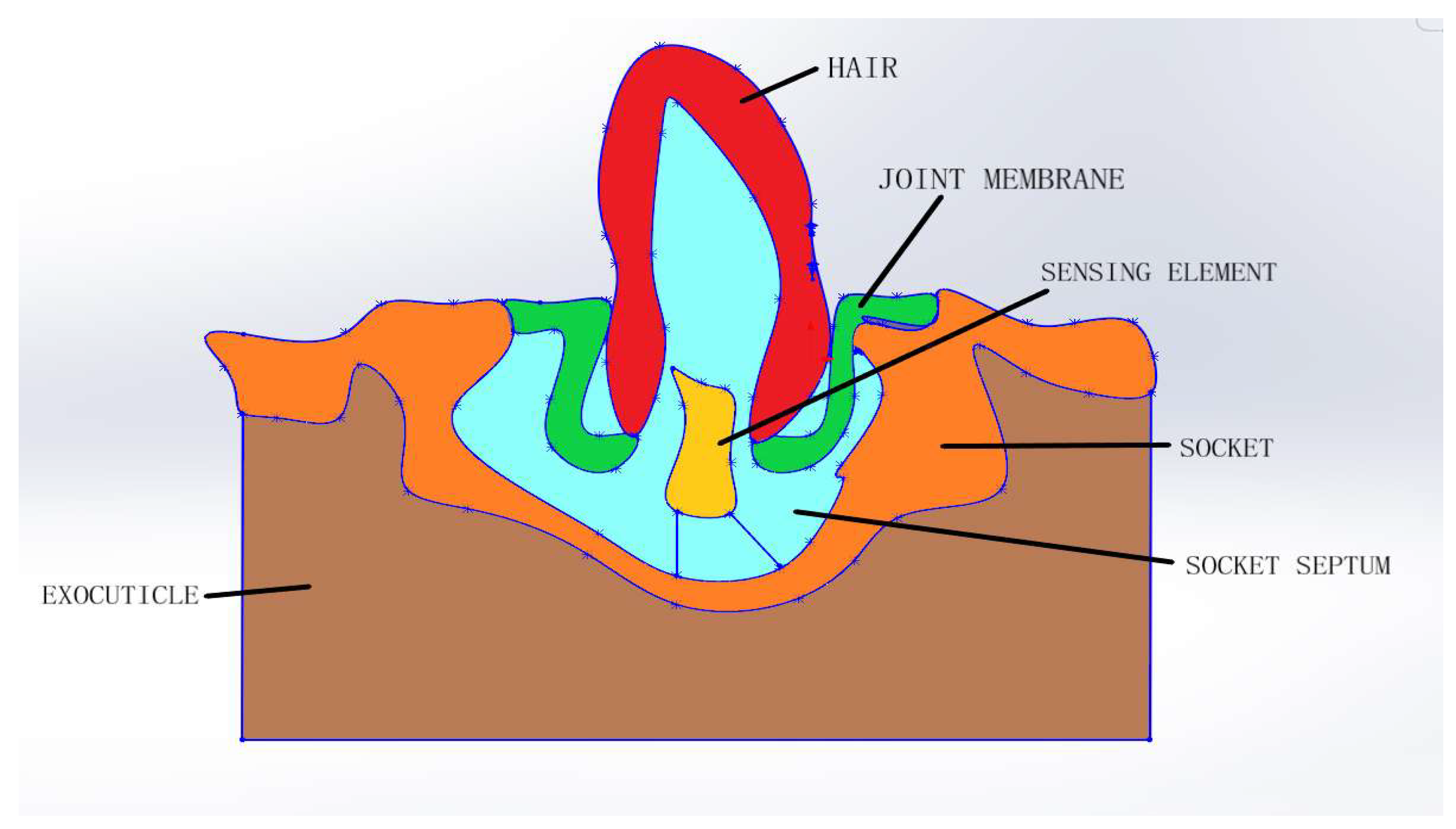
3.2. Internal Morphology of the Sensilla
4. Discussion
4.1. Development of the Sensilla
4.2. Functioning of Sensilla
4.3. Design of Artificial Mechanosensors
5. Conclusions
References
- George, A.; Nagy, B.A.L. Morphology, distribution, and ultrastructural differences of sensilla trichodea and basiconica on the antennae of the oriental fruit moth, Grapholitha molesta (Busck) (Lepidoptera: Tortricidae). Int. J. Insect Morphol. Embryol. 1984, 13, 157–170. [Google Scholar] [CrossRef]
- Shimozawa, T.; Kanou, M. The aerodynamics and sensory physiology of range fractionation in the cereal filiform sensilla of the cricket Gryllus bimaculatus. J. Comp. Physiol. 1984, 155, 495–505. [Google Scholar] [CrossRef]
- Dey, S.; Hooroo, R.N.; Wankhar, D. Scanning electron microscopic studies of the external morphology of sensilla on the legs of a butterfly, Graphium sarpedon (Lepidoptera—Papillionidae). Micron 1995, 26, 367–376. [Google Scholar] [CrossRef]
- Dijkstra, M.; van Baar, J.J.; Wiegerink, R.J.; Lammerink, T.S.J.; de Boer, J.H.; Krijnen, G.J.M. Artificial sensory hairs based on the flow sensitive receptor hairs of crickets. J. Micromech. Microeng. 2005, 15, S132–S138. [Google Scholar] [CrossRef]
- Dagamseh, A.M.K. Estimation of squeeze film damping in artificial hair-sensor towards the detection-limit of crickets’ hairs. Microsyst. Technol. 2014, 20, 963–970. [Google Scholar] [CrossRef]
- Krijnen, G.J.M.; Droogendijk, H.; Dagamseh, A.M.K.; Jaganatharaja, R.K.; Casas, J. Crickets as Bio-Inspiration for MEMS-Based Flow-Sensing. In Flow Sensing in Air and Water; Bleckmann, H., Mogdans, J., Coombs, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 459–488. [Google Scholar] [CrossRef]
- Krijnen, G.J.M.; Lammerink, T.; Wiegerink, R. Learning from crickets: Artificial hair-sensor array developments. In Proceedings of the SENSORS 2010 IEEE, Kona, HI, USA, 1–4 November 2010; pp. 2218–2223. [Google Scholar] [CrossRef]
- Krijnen, G.; Lammerink, T.; Wiegerink, R.; Casas, J. Cricket Inspired Flow-Sensor Arrays. In Proceedings of the SENSORS 2007 IEEE, Atlanta, GA, USA, 28–31 October 2007; pp. 539–546. [Google Scholar] [CrossRef]
- Droogendijk, H.; Casas, J.; Steinmann, T.; Krijnen, G.J. Performance assessment of bio-inspired systems: Flow sensing MEMS hairs. Bioinspir. Biomim. 2014, 10, 016001. [Google Scholar] [CrossRef] [PubMed]
- Droogendijk, H. Bio-Inspired MEMS Flow and Inertial Sensors; University of Twente: Enschede, The Netherlands, 2014. [Google Scholar] [CrossRef][Green Version]
- di Giulio, A.; Maurizi, E.; Valerio, M.; Stacconi, R.; Romani, R. Functional structure of antennal sensilla in the myrmecophilous beetle Paussus favieri (Coleoptera, Carabidae, Paussini). Micron 2012, 43, 705–719. [Google Scholar] [CrossRef] [PubMed]
- Keil, T.A. Functional morphology of insect mechanoreceptors. Microsc. Res. Tech. 1997, 39, 506–531. [Google Scholar] [CrossRef]
- Letourneau, P.C.; Condic, M.L.; Snow, D.M. Interactions of developing neurons with the extracellular matrix. J. Neurosci. 1994, 14, 915–928. [Google Scholar] [CrossRef] [PubMed]
- Orgogozo, V.; Grueber, W.B. FlyPNS, A database of the Drosophila embryonic and larval peripheral nervous system. BMC Dev. Biol. 2005, 5, 4. [Google Scholar] [CrossRef] [PubMed]
- Giangrande, A.; Palka, J. Genes involved in the development of the peripheral nervous system of Drosophila. Semin. Cell Biol. 1990, 1, 197–209. [Google Scholar] [PubMed]
- Gnatzy, W.; Weber, K.M. Tormogen cell and receptor-lymph space in insect olfactory sensilla. Cell Tissue Res. 1978, 189, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Fred, T.; Hartley, D.A. Inhibition of cell fate in Drosophila by Enhancer of split genes. Mech. Dev. 1995, 51, 305–315. [Google Scholar]
- Barth, F.G. Spider mechanoreceptors. Curr. Opin. Neurobiol. 2004, 4, 415–422. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chakilam, S.; Li, D.T.; Xi, Z.C.; Gaidys, R.; Lupeikiene, A. Morphological Study of Insect Mechanoreceptors to Develop Artificial Bio-Inspired Mechanosensors. Eng. Proc. 2020, 2, 70. https://doi.org/10.3390/ecsa-7-08199
Chakilam S, Li DT, Xi ZC, Gaidys R, Lupeikiene A. Morphological Study of Insect Mechanoreceptors to Develop Artificial Bio-Inspired Mechanosensors. Engineering Proceedings. 2020; 2(1):70. https://doi.org/10.3390/ecsa-7-08199
Chicago/Turabian StyleChakilam, Shashikanth, Dan Ting Li, Zhang Chuan Xi, Rimvydas Gaidys, and Audrone Lupeikiene. 2020. "Morphological Study of Insect Mechanoreceptors to Develop Artificial Bio-Inspired Mechanosensors" Engineering Proceedings 2, no. 1: 70. https://doi.org/10.3390/ecsa-7-08199
APA StyleChakilam, S., Li, D. T., Xi, Z. C., Gaidys, R., & Lupeikiene, A. (2020). Morphological Study of Insect Mechanoreceptors to Develop Artificial Bio-Inspired Mechanosensors. Engineering Proceedings, 2(1), 70. https://doi.org/10.3390/ecsa-7-08199




