Removal of Methylene Blue from Aqueous Solution by Application of Plant-Based Coagulants †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Analytic Techniques
2.3. Plant-Based Coagulant Preparation
2.4. Characterization of Plant-Based Coagulants
2.5. Coagulation–Flocculation–Decantation Experimental Setup
- (1)
- The pH was varied (3.0, 5.0, 7.0, 9.0 and 11.0) under the operational conditions: [MB] = 50 mg/L, [coagulant] = 1.0 g/L, fast mix 150 rpm/3 min, slow mix 20 rpm/20 min, T = 298 K, V = 250 mL, sedimentation time = 30 min;
- (2)
- After determining the best pH in (1), the dosage of coagulant was varied (0.1, 0.5, 1.0 and 2.0 g/L) under the operational conditions: [MB] = 50 mg/L, fast mix 150 rpm/3 min, slow mix 20 rpm/20 min, T = 298 K, V = 250 mL, sedimentation time = 30 min;
- (3)
- With the best pH (1) and coagulant dosage (2), the mixing conditions were tested by varying the fast and slow mix under the operational conditions: [MB] = 50 mg/L, T = 298 K, V = 250 mL, sedimentation time = 30 min;
- (4)
- Under pre-determinate values of pH, coagulant dosage and mixing conditions obtained in (1)–(3), the concentration of activated sodium bentonite (0.1, 0.5, 1.0 and 2.0 g/L) as a flocculant was varied under the operational conditions: [MB] = 50 mg/L, T = 298 K, V = 250 mL, sedimentation time = 30 min.
3. Results and Discussion
Coagulation–Flocculation–Decantation Experiments
4. Conclusions
- (1)
- The plant-based coagulants are carbon-based materials with porous structures that can adsorb the contaminants;
- (2)
- Under the best operational conditions, the plant-based coagulants achieve a high removal of MB from aqueous solution;
- (3)
- The addition of bentonite significantly increases the efficiency of the CFD process.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liang, J.; Ning, X.; Kong, M.; Liu, D.; Wang, G.; Cai, H.; Sun, J.; Zhang, Y.; Lu, X.; Yuan, Y. Elimination and Ecotoxicity Evaluation of Phthalic Acid Esters from Textile-Dyeing Wastewater. Environ. Pollut. 2017, 231, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Jorge, N.; Teixeira, A.R.; Matos, C.C.; Lucas, M.S.; Peres, J.A. Combination of Coagulation–Flocculation–Decantation and Ozonation Processes for Winery Wastewater Treatment. Int. J. Environ. Res. Public Health 2021, 18, 8882. [Google Scholar] [CrossRef] [PubMed]
- Tie, J.; Jiang, M.; Li, H.; Zhang, S.; Zhang, X. A Comparison between Moringa Oleifera Seed Presscake Extract and Polyaluminum Chloride in the Removal of Direct Black 19 from Synthetic Wastewater. Ind. Crop. Prod. 2015, 74, 530–534. [Google Scholar] [CrossRef]
- Martins, R.B.; Jorge, N.; Lucas, M.S.; Raymundo, A.; Barros, A.I.; Peres, J.A. Food By-Product Valorization by Using Plant-Based Coagulants Combined with AOPs for Agro-Industrial Wastewater Treatment. Int. J. Environ. Res. Public Health 2022, 19, 4134. [Google Scholar] [CrossRef] [PubMed]
- Zavoi, S.; Fetea, F.; Ranga, F.; Pop, R.M.; Baciu, A.; Socaciu, C. Comparative Fingerprint and Extraction Yield of Medicinal Herb Phenolics with Hepatoprotective Potential, as Determined by UV-Vis and FT-MIR Spectroscopy. Not. Bot. Horti Agrobot. Cluj-Napoca 2011, 39, 82–89. [Google Scholar] [CrossRef] [Green Version]
- Zimmermann, B.; Bağcıoğlu, M.; Tafinstseva, V.; Kohler, A.; Ohlson, M.; Fjellheim, S. A High-Throughput FTIR Spectroscopy Approach to Assess Adaptive Variation in the Chemical Composition of Pollen. Ecol. Evol. 2017, 7, 10839–10849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vunain, E.; Mike, P.; Mpeketula, G.; Monjerezi, M.; Etale, A. Evaluation of Coagulating Efficiency and Water Borne Pathogens Reduction Capacity of Moringa Oleifera Seed Powder for Treatment of Domestic Wastewater from Zomba, Malawi. J. Environ. Chem. Eng. 2019, 7, 103118. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Beltrán-Heredia, J.; Sánchez-Martín, J.; Delgado-Regalado, A. Removal of Carmine Indigo Dye with Moringa Oleifera Seed Extract. Ind. Eng. Chem. Res. 2009, 48, 6512–6520. [Google Scholar] [CrossRef]
- Sánchez-Martín, J.; Beltrán-Heredia, J.; Peres, J.A. Improvement of the Flocculation Process in Water Treatment by Using Moringa Oleifera Seeds Extract. Braz. J. Chem. Eng. 2012, 29, 495–501. [Google Scholar] [CrossRef] [Green Version]
- Sanghi, R.; Bhattacharya, B. Adsorption-Coagulation for the Decolorisation of Textile Dye Solutions. Water Qual. Res. J. 2003, 38, 553–562. [Google Scholar] [CrossRef]
- Jorge, N.; Teixeira, A.R.; Guimarães, V.; Lucas, M.S.; Peres, J.A. Treatment of Winery Wastewater with a Combination of Adsorption and Thermocatalytic Processes. Processes 2022, 10, 75. [Google Scholar] [CrossRef]
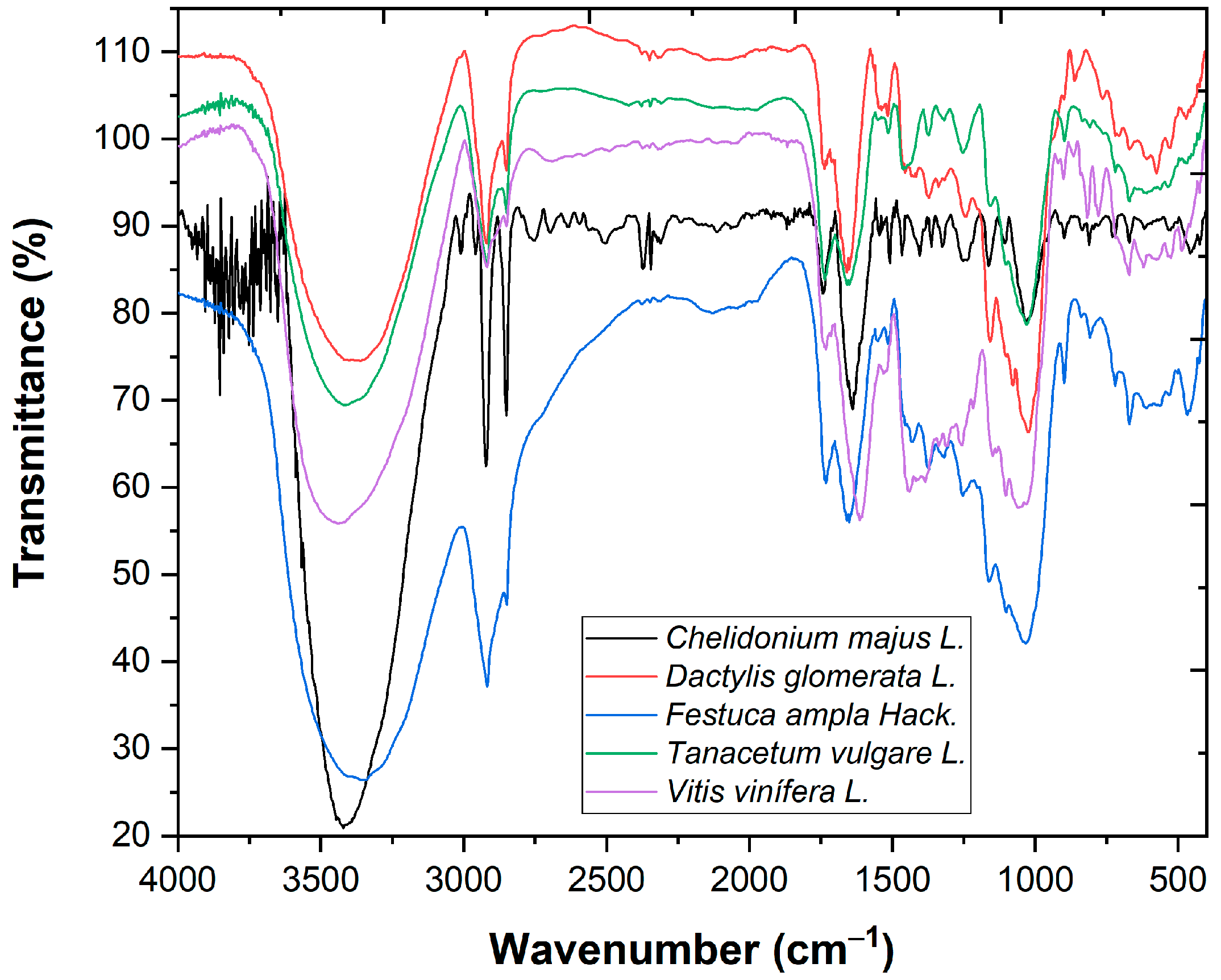
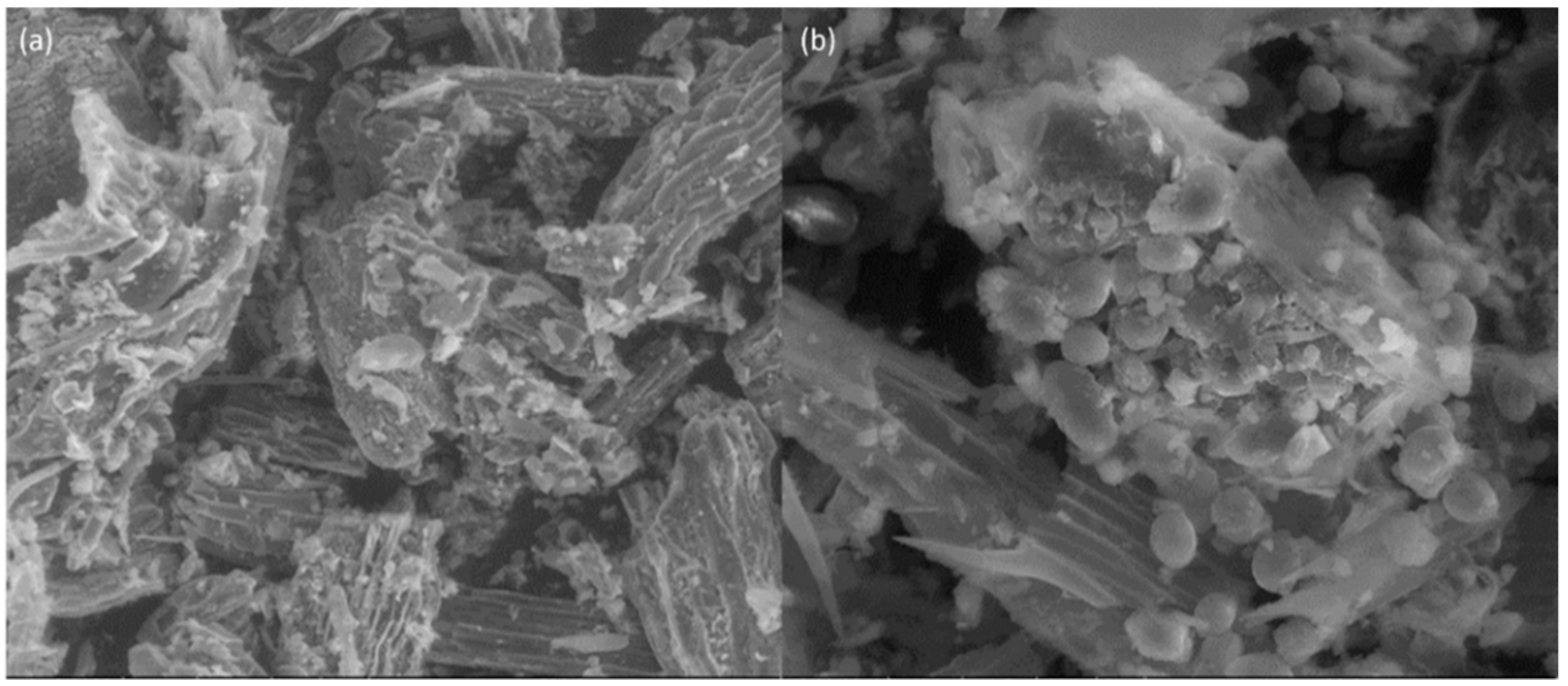

| Plant Specie | Sub Specie | Part Collected | Herbarium Number |
|---|---|---|---|
| Chelidonium majus L. | Seed | ||
| Dactylis glomerata L. | lusitanica Stebbins et Zohary | Seed | HVR22101 |
| Festuca ampla Hack. | ampla | Seed | HVR22102 |
| Tanacetum vulgare L. | Seed | HVR22099 | |
| Vitis vinifera L. | Rachis |
| Coagulants | SBET (m2/g) |
|---|---|
| C. majus | 0.05 |
| D. glomerata | 0.06 |
| F. ampla | 0.18 |
| T. vulgare | 0.03 |
| V. vinifera | 0.50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jorge, N.; Teixeira, A.R.; Marchão, L.; Tavares, P.B.; Lucas, M.S.; Peres, J.A. Removal of Methylene Blue from Aqueous Solution by Application of Plant-Based Coagulants. Eng. Proc. 2022, 19, 38. https://doi.org/10.3390/ECP2022-12659
Jorge N, Teixeira AR, Marchão L, Tavares PB, Lucas MS, Peres JA. Removal of Methylene Blue from Aqueous Solution by Application of Plant-Based Coagulants. Engineering Proceedings. 2022; 19(1):38. https://doi.org/10.3390/ECP2022-12659
Chicago/Turabian StyleJorge, Nuno, Ana R. Teixeira, Leonilde Marchão, Pedro B. Tavares, Marco S. Lucas, and José A. Peres. 2022. "Removal of Methylene Blue from Aqueous Solution by Application of Plant-Based Coagulants" Engineering Proceedings 19, no. 1: 38. https://doi.org/10.3390/ECP2022-12659
APA StyleJorge, N., Teixeira, A. R., Marchão, L., Tavares, P. B., Lucas, M. S., & Peres, J. A. (2022). Removal of Methylene Blue from Aqueous Solution by Application of Plant-Based Coagulants. Engineering Proceedings, 19(1), 38. https://doi.org/10.3390/ECP2022-12659









