Development of a Biosensor for the Early Detection of Tuberculous Meningitis in Infants †
Abstract
1. Introduction
2. Materials and Methodology
2.1. Biosensor Fabrication
2.2. Chip Functionalization and Immobilization
2.3. Image Capturing and Processing
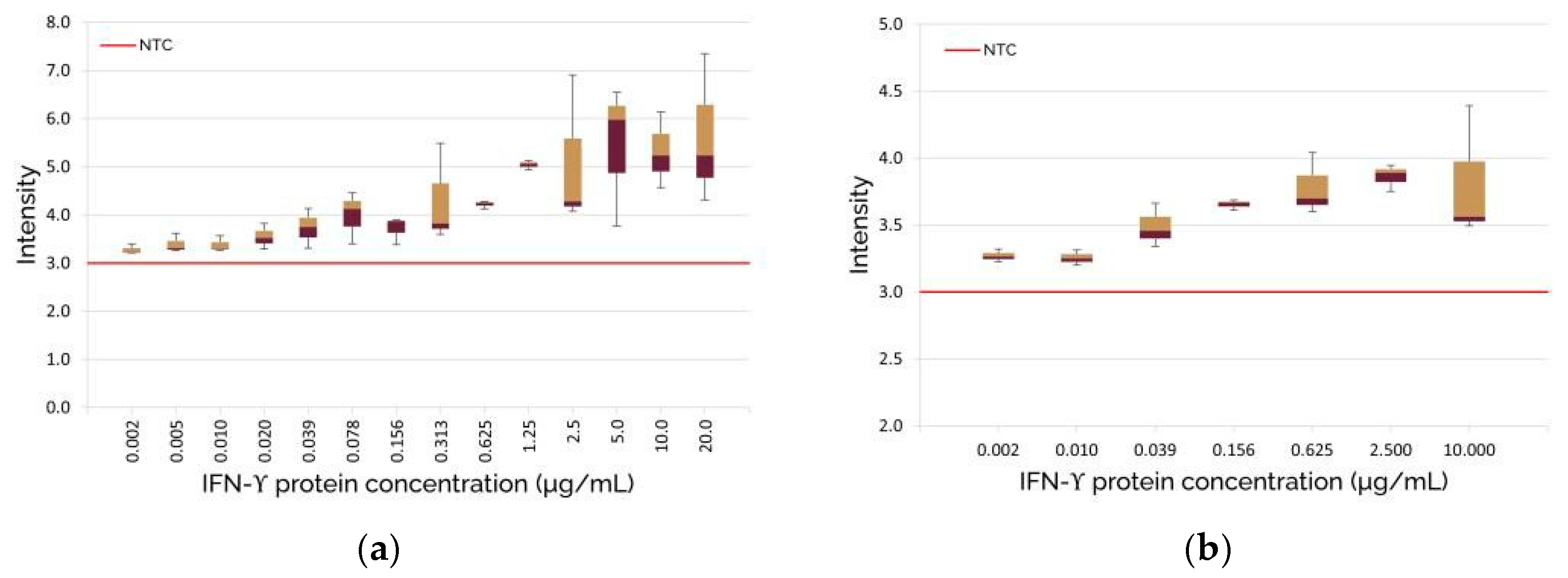
3. Results and Discussion
3.1. Immobilization Verification
3.2. Optical Biosensor Verification and Image Processing
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Slane, V.H.; Unakal, C.G. Tuberculous Meningitis. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK541015/ (accessed on 2 August 2024).
- Rich, A.R.; McCordick, H.A. The pathogenesis of tuberculous meningitis. Bull. John Hopkins Hosp. 1933, 52, 5–37. [Google Scholar]
- du Preez, K.; Jenkins, H.E.; Donald, P.R.; Solomons, R.S.; Graham, S.M.; Schaaf, H.S.; Starke, J.R.; Hesseling, A.C.; Seddon, J.A. Tuberculous Meningitis in Children: A Forgotten Public Health Emergency. Front. Neurol. 2022, 13, 751133. [Google Scholar] [CrossRef] [PubMed]
- Dodd, P.J.; Osman, M.; Cresswell, F.V.; Stadelman, A.M.; Lan, N.H.; Thuong, N.T.T.; Muzyamba, M.; Glaser, L.; Dlamini, S.S.; Seddon, J.A. The global burden of tuberculous meningitis in adults: A modelling study. PLoS Glob. Public Health 2021, 1, e0000069. [Google Scholar] [CrossRef] [PubMed]
- Solomons, R.; Grantham, M.; Marais, B.J.; van Toorn, R. IMCI indicators of childhood TBM at primary health care level in the Western Cape Province of South Africa. Int. J. Tuberc. Lung Dis. 2016, 20, 1309–1313. [Google Scholar] [CrossRef] [PubMed]
- Marx, G.E.; Chan, E.D. Tuberculous meningitis: Diagnosis and treatment overview. Tuberc. Res. Treat. 2011, 2011, 798764. [Google Scholar] [CrossRef] [PubMed]
- Nalugwa, T.; Shete, P.B.; Nantale, M.; Farr, K.; Ojok, C.; Ochom, E.; Mugabe, F.; Joloba, M.; Dowdy, D.W.; Moore, D.A.J.; et al. Challenges with scale-up of GeneXpert MTB/RIF® in Uganda: A health systems perspective. BMC Health Serv. Res. 2020, 20, 162. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. Meeting Report: High-Priority Target Product Profiles for New Tuberculosis Diagnostics: Report of a Consensus Meeting; World Health Organisation: Geneva, Switzerland, 2014. [Google Scholar]
- Manyelo, C.M.; Solomons, R.S.; Snyders, C.I.; Manngo, P.M.; Mutavhatsindi, H.; Kriel, B.; Stanley, K.; Walzl, G.; Chegou, N.N. Application of Cerebrospinal Fluid Host Protein Biosignatures in the Diagnosis of Tuberculous Meningitis in Children from a High Burden Setting. Mediat. Inflamm. 2019, 2019, 7582948. [Google Scholar] [CrossRef] [PubMed]
- Manyelo, C.M.; Solomons, R.S.; Snyders, C.I.; Kidd, M.; Kooblal, Y.; Leukes, V.N.; Claassen, C.; Roos, K.; Stanley, K.; Walzl, G.; et al. Validation of host cerebrospinal fluid protein biomarkers for early diagnosis of tuberculous meningitis in children: A replication and new biosignature discovery study. Biomarkers 2022, 27, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Mouse Anti-Human IFN-γ-UNLB (A35). Available online: https://www.southernbiotech.com/mouse-anti-human-ifn-g-unlb-a35-10113-01 (accessed on 29 January 2025).
- Recombinant Human IFN Gamma Protein. Available online: https://www.sinobiological.com/recombinant-proteins/human-interferon-gamma-11725-hnas (accessed on 29 January 2025).
- Mouse Anti-Human IFN-γ-FITC (B27). Available online: https://www.southernbiotech.com/mouse-anti-human-ifn-g-fitc-b27-10114-02 (accessed on 29 January 2025).

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.; Perold, W.J.; Chegou, N.N. Development of a Biosensor for the Early Detection of Tuberculous Meningitis in Infants. Eng. Proc. 2025, 109, 12. https://doi.org/10.3390/engproc2025109012
Kim D, Perold WJ, Chegou NN. Development of a Biosensor for the Early Detection of Tuberculous Meningitis in Infants. Engineering Proceedings. 2025; 109(1):12. https://doi.org/10.3390/engproc2025109012
Chicago/Turabian StyleKim, Dabin, Willem Jacobus Perold, and Novel N. Chegou. 2025. "Development of a Biosensor for the Early Detection of Tuberculous Meningitis in Infants" Engineering Proceedings 109, no. 1: 12. https://doi.org/10.3390/engproc2025109012
APA StyleKim, D., Perold, W. J., & Chegou, N. N. (2025). Development of a Biosensor for the Early Detection of Tuberculous Meningitis in Infants. Engineering Proceedings, 109(1), 12. https://doi.org/10.3390/engproc2025109012





