Deep Learning Approach for Breast Cancer Detection Using UNet and CNN in Ultrasound Imaging †
Abstract
1. Introduction
2. Literature Review
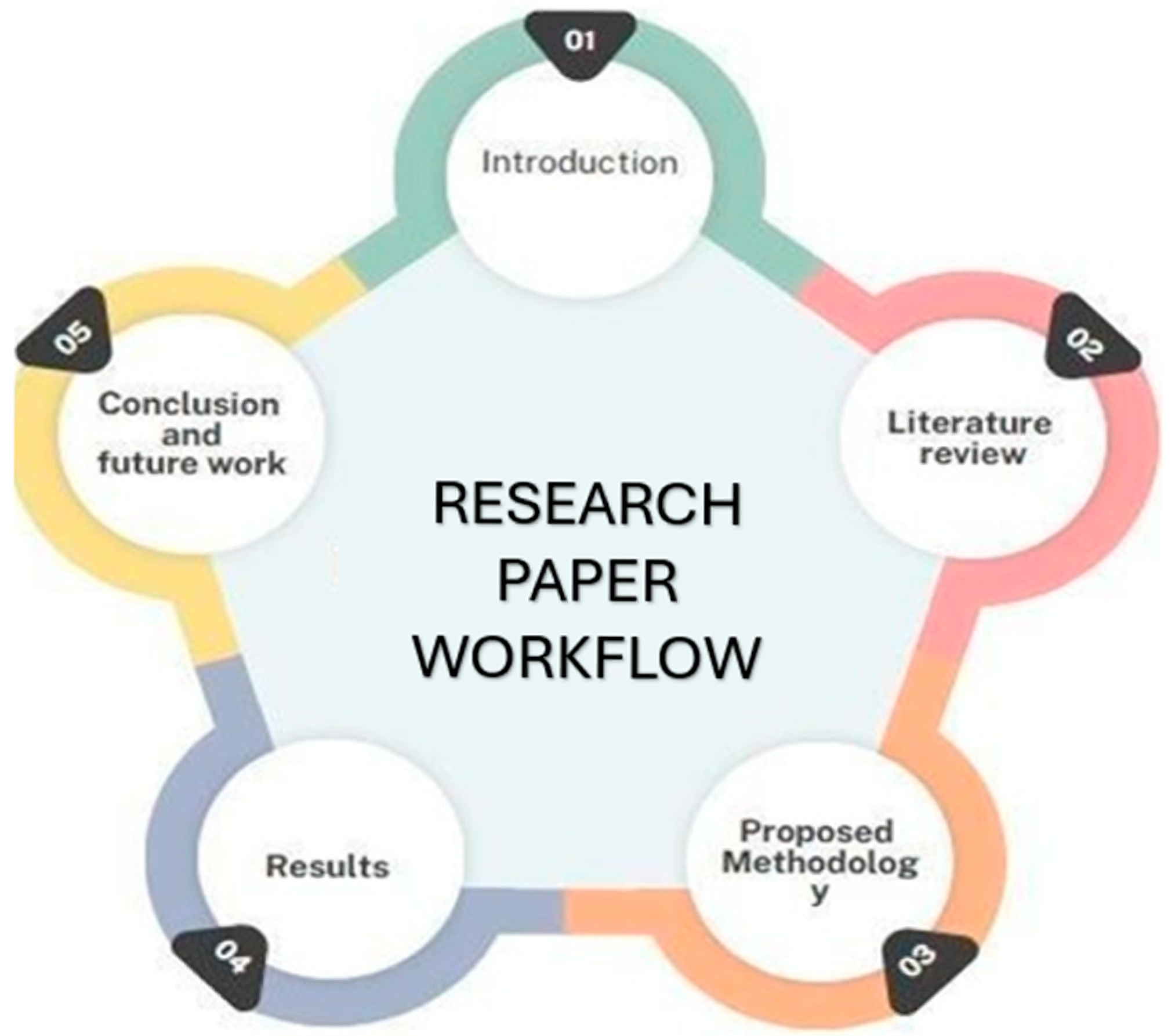
3. Methodology
- Decision TreeThe dataset was divided relative to feature values utilizing a decision tree classifier, thereby creating a tree structure to categorize obesity levels. The dataset wasperiodically divided into subgroups by the tree according to the characteristics that decrease impurity or optimize information gain. Information gain, where • H(X) = entropy of the dataset T. • Xv = subset of T for which feature Z has value v. Entropy: • Pi = proportion of class i in dataset X. Figure 2 shows how information gain is calculated.

- Random ForestRandom forest is an ensemble learning method that builds several decision trees during training and chooses the most common class for classification tasks or average predictions for regression tasks to obtain the final result. Random forest formula:where prediction of the decision tree for input a. Number of decision trees.
- K-Nearest Neighbors (K-NNs)The K-NN algorithm classifies a data point based on most classes of its k-nearest neighbors. Distance of metric: the degree of resemblance between data points is determined using the Euclidean distance:where • A and B = two data points in n-dimensional space.
- Naïve BayesAssuming feature independence, the naïve Bayes classification technique is based on Bayes’ theorem. It works well with large data and uses feature likelihoods to estimate the likelihood that a data point belongs to a class.where
- P(A|K) = posterior probability of class A given feature vector K;
- P(K|A) = likelihood of K given class A;
- P(A) = prior probability of class A;
- P(K) = marginal probability of K.
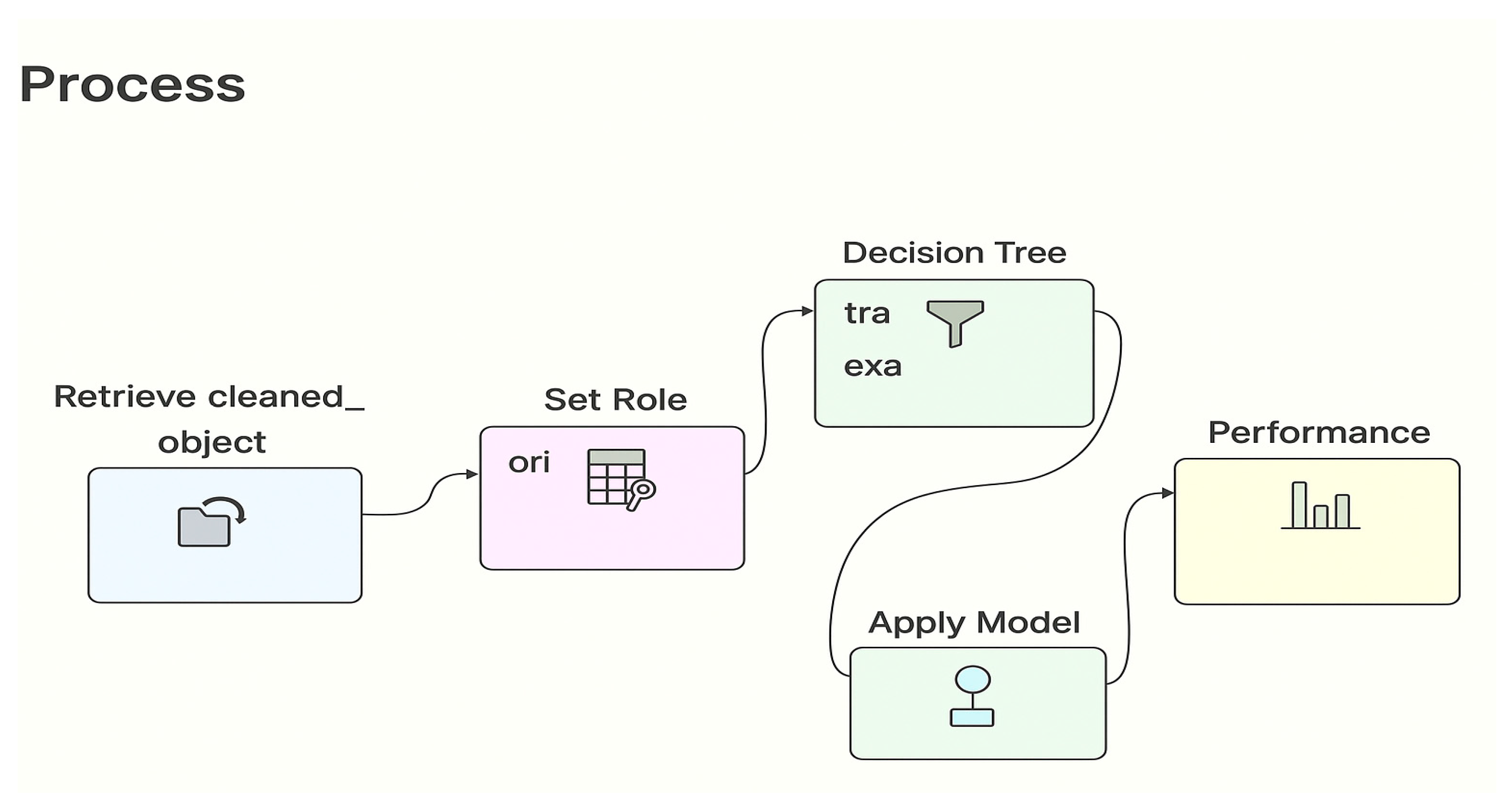
3.1. Framework
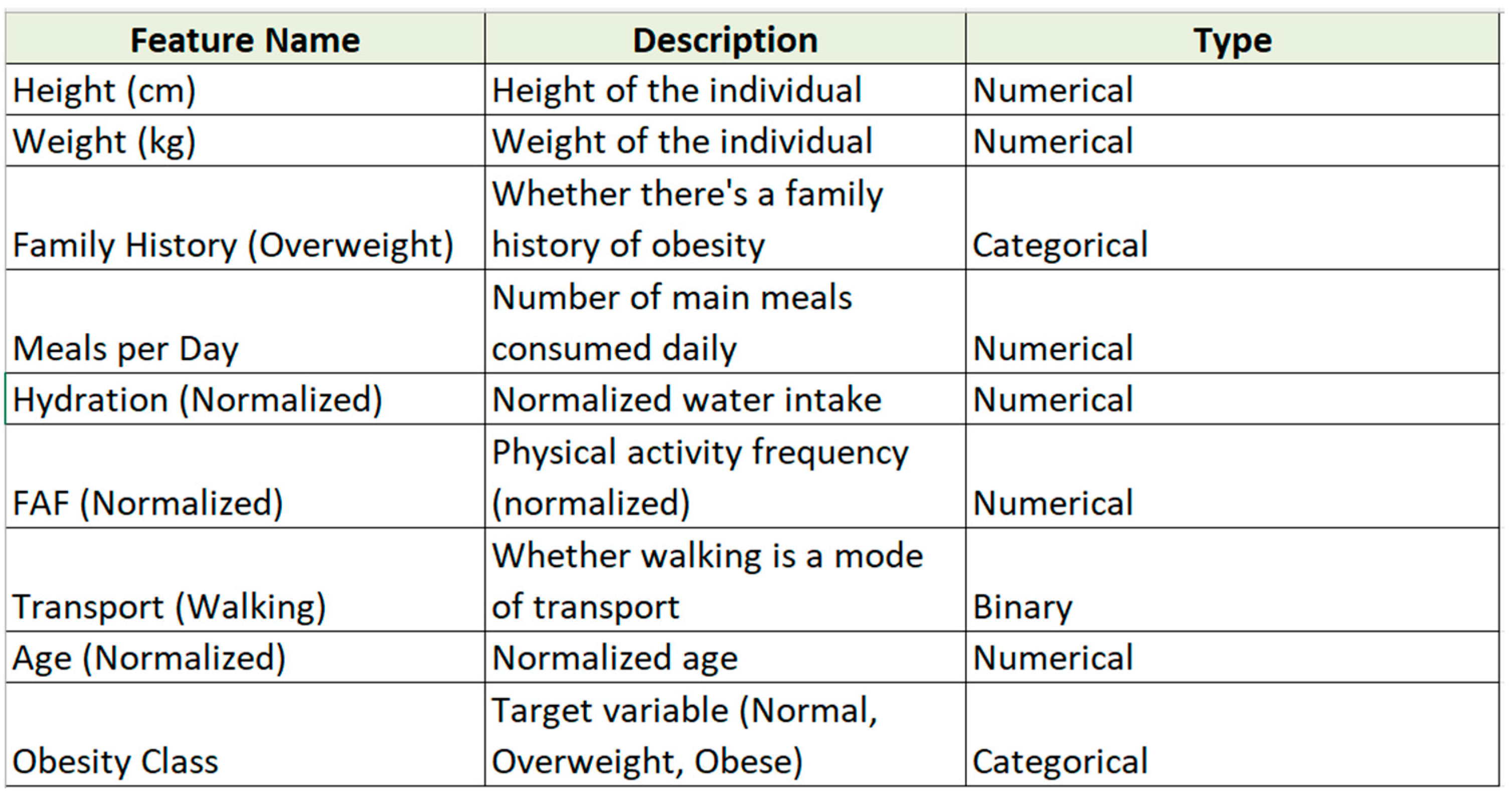
3.2. Attribute with Description
3.3. Replace Missing Values
3.4. Split Data
3.5. Machine Learning
4. Results
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jeon, J.; Lee, S.; Oh, C. Age-specific risk factors for the prediction of obesity using a machine learning approach. Front. Public Health 2023, 10, 998782. [Google Scholar] [CrossRef] [PubMed]
- Colmenarejo, G. Machine learning models to predict childhood and adolescent obesity: A review. Nutrients 2020, 12, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.; Kim, M.; Yoon, J.; Lee, S.; Youm, S. Machine learning-based obesity classification considering 3D body scanner measurements. Sci. Rep. 2023, 13, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Kumar, R.; Gupta, M. Predicting risk of obesity and meal planning to reduce the obese in adulthood using artificial intelligence. Endocrine 2022, 78, 458–469. [Google Scholar] [CrossRef] [PubMed]
- Osadchiy, V.; Bal, R.; Mayer, E.A.; Kunapuli, R.; Dong, T.; Vora, P.; Petrasek, D.; Liu, C.; Stains, J.; Gupta, A. Machine learning model to predict obesity using gut metabolite and brain microstructure data. Sci. Rep. 2023, 13, 5488. [Google Scholar] [CrossRef] [PubMed]
- Cheng, E.; Steinhardt, R.; Miled, Z. Predicting childhood obesity using machine learning: Practical considerations. BioMedInformatics 2022, 2, 184–203. [Google Scholar] [CrossRef]
- Sun, Z.; Yuan, Y.; Farrahi, V.; Herold, F.; Xia, Z.; Xiong, X.; Qiao, Z.; Shi, Y.; Yang, Y.; Qi, K.; et al. Using interpretable machine learning methods to identify the relative importance of lifestyle factors for overweight and obesity in adults: Pooled evidence from CHNS and NHANES. BMC Public Health 2024, 24, 3034. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Tawfik, H. Machine learning approach for the early prediction of the risk of overweight and obesity in young people. In International Conference on Computational Science (ICCS); Springer International Publishing: Cham, Switzerland, 3 June 2020; pp. 523–535. [Google Scholar]
- Kim, H.; Lim, D.; Kim, Y. Classification and prediction on the effects of nutritional intake on overweight/obesity, dyslipidemia, hypertension and type 2 diabetes mellitus using deep learning model: 4–7th Korea National Health and Nutrition Examination Survey. Int. J. Environ. Res. Public Health 2021, 18, 5597. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Yang, S.; Zeng, Y.; Ye, C.; Chang, X.; Wu, S. Visualization obesity risk prediction system based on machine learning. Sci. Rep. 2024, 14, 22424. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Lin, S.Y.; Liu, J.; Liu, S.; Zhang, J.; Nie, P.; Fuemmeler, B.F.; Wang, Y.; Xue, H. Does physical activity predict obesity—A machine learning and statistical method-based analysis. Int. J. Environ. Res. Public Health 2021, 18, 3966. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Forrest, C.; Lê-Scherban, F.; Masino, A. Prediction of early childhood obesity with machine learning and electronic health record data. Int. J. Med. Inform. 2021, 150, 104454. [Google Scholar] [CrossRef] [PubMed]
- Diwaker, C.; Tomar, P.; Solanki, A.; Nayyar, A.; Jhanjhi, N.Z.; Abdullah, A.; Supramaniam, M. A new model for predicting component-based software reliability using soft computing. IEEE Access 2019, 7, 147191–147203. [Google Scholar] [CrossRef]
- Kok, S.H.; Abdullah, A.; Jhanjhi, N.Z.; Supramaniam, M. A review of intrusion detection system using machine learning approach. Int. J. Eng. Res. Technol. 2019, 12, 8–15. [Google Scholar]
- Airehrour, D.; Gutierrez, J.; Ray, S.K. GradeTrust: A secure trust based routing protocol for MANETs. In Proceedings of the 25th International Telecommunication Networks and Applications Conference (ITNAC), Sydney, Australia, 18–20 November 2015; pp. 65–70. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ch, R.; Naresh, U.; Malik, A.; Hattamurrahman, M.P.S. Deep Learning Approach for Breast Cancer Detection Using UNet and CNN in Ultrasound Imaging. Eng. Proc. 2025, 107, 77. https://doi.org/10.3390/engproc2025107077
Ch R, Naresh U, Malik A, Hattamurrahman MPS. Deep Learning Approach for Breast Cancer Detection Using UNet and CNN in Ultrasound Imaging. Engineering Proceedings. 2025; 107(1):77. https://doi.org/10.3390/engproc2025107077
Chicago/Turabian StyleCh, Ravikumar, Usikela Naresh, Arun Malik, and M. Putra Sani Hattamurrahman. 2025. "Deep Learning Approach for Breast Cancer Detection Using UNet and CNN in Ultrasound Imaging" Engineering Proceedings 107, no. 1: 77. https://doi.org/10.3390/engproc2025107077
APA StyleCh, R., Naresh, U., Malik, A., & Hattamurrahman, M. P. S. (2025). Deep Learning Approach for Breast Cancer Detection Using UNet and CNN in Ultrasound Imaging. Engineering Proceedings, 107(1), 77. https://doi.org/10.3390/engproc2025107077





