Fabrication and Characterization of Perovskite Solar Cells Using Copper Phthalocyanine Complex with Tetracyanoquinodimethane †
Abstract
:1. Introduction
2. Materials and Methods
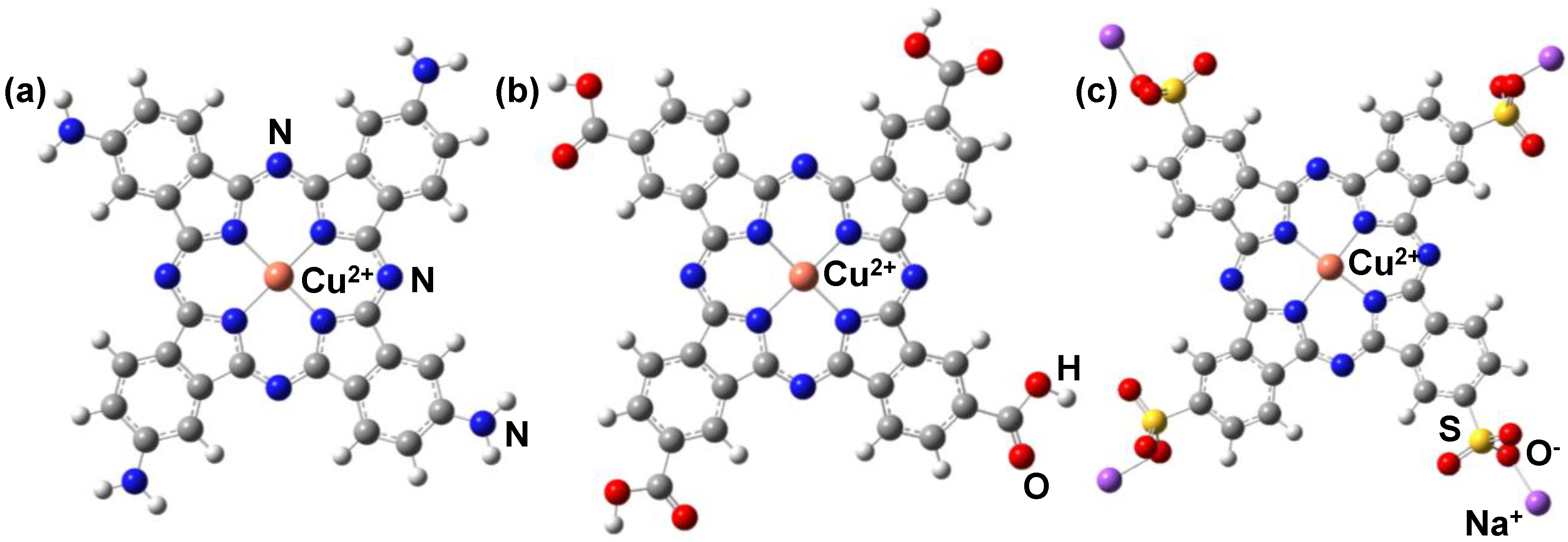
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yoo, J.J.; Seo, G.; Chua, M.R.; Park, T.G.; Lu, Y.; Rotermund, F.; Kim, Y.-K.; Moon, C.S.; Jeon, N.J.; Correa-Baena, J.-P.; et al. Efficient perovskite solar cells via improved carrier management. Nature 2021, 590, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.; Kim, M.; Seo, J.; Lu, H.; Ahlawat, P.; Mishra, A.; Yang, Y.; Hope, M.A.; Eickemeyer, F.T.; Kim, M.; et al. Pseudo-halide anion engineering for α-FAPbI3 perovskite solar cells. Nature 2021, 592, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Zhu, C.; Chen, M.; Jiang, C.; Guo, J.; Feng, Y.; Dai, Z.; Yadavalli, S.K.; Hu, M.; Cao, X.; et al. Interpenetrating interfaces for efficient perovskite solar cells with high operational stability and mechanical robustness. Nat. Commun. 2021, 12, 973. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Jung, H.J.; Park, I.J.; Larson, B.W.; Dunfield, S.P.; Xiao, C.; Kim, J.; Tong, J.; Boonmongkolras, P.; Ji, S.G.; et al. Efficient, stable silicon tandem cells enabled by anion-engineered wide-bandgap perovskites. Science 2020, 368, 155–160. [Google Scholar] [CrossRef]
- Gao, B.; Meng, J. RbCs(MAFA)PbI3 perovskite solar cell with 22.81% efficiency using the precise ions cascade regulation. Appl. Surf. Sci. 2020, 530, 147240. [Google Scholar] [CrossRef]
- Sun, P.-P.; Kripalani, D.R.; Chi, W.; Snyder, S.A.; Zhou, K. High carrier mobility and remarkable photovoltaic performance of two-dimensional Ruddlesden–Popper organic–inorganic metal halides (PA)2(MA)2M3I10 for perovskite solar cell applications. Mater. Today 2021, 47, 45–52. [Google Scholar] [CrossRef]
- Liu, Z.; Qiu, L.; Ono, L.K.; He, S.; Hu, Z.; Jiang, M.; Tong, G.; Wu, Z.; Jiang, Y.; Son, D.-Y.; et al. A holistic approach to interface stabilization for efficient perovskite solar modules with over 2,000-hour operational stability. Nat. Energy 2020, 5, 596–604. [Google Scholar] [CrossRef]
- Nishi, K.; Oku, T.; Kishimoto, T.; Ueoka, N.; Suzuki, A. Photovoltaic Characteristics of CH3NH3PbI3 Perovskite Solar Cells Added with Ethylammonium Bromide and Formamidinium Iodide. Coatings 2020, 10, 410. [Google Scholar] [CrossRef] [Green Version]
- Lyu, M.; Park, N.-G. Effect of Additives AX (A = FA, MA, Cs, Rb, NH4, X = Cl, Br, I) in FAPbI3 on Photovoltaic Parameters of Perovskite Solar Cells. Sol. RRL 2020, 4, 202000331. [Google Scholar] [CrossRef]
- Kim, G.; Min, H.; Lee, K.S.; Lee, D.Y.; Yoon, S.M.; Seok, S.I. Impact of strain relaxation on performance of α-formamidinium lead iodide perovskite solar cells. Science 2020, 370, 108–112. [Google Scholar] [CrossRef]
- Gao, L.; Li, X.; Liu, Y.; Fang, J.; Huang, S.; Spanopoulos, I.; Li, X.; Wang, Y.; Chen, L.; Yang, G.; et al. Incorporated Guanidinium Expands the CH3NH3PbI3 Lattice and Enhances Photovoltaic Performance. ACS Appl. Mater. Interfaces 2020, 12, 43885–43891. [Google Scholar] [CrossRef] [PubMed]
- Chavan, R.D.; Prochowicz, D.; Tavakoli, M.M.; Yadav, P.; Hong, C.K. Surface Treatment of Perovskite Layer with Guanidinium Iodide Leads to Enhanced Moisture Stability and Improved Efficiency of Perovskite Solar Cells. Adv. Mater. Interfaces 2020, 7, 2000105. [Google Scholar] [CrossRef]
- Kishimoto, T.; Oku, T.; Suzuki, A.; Ueoka, N. Additive Effects of Guanidinium Iodide on CH3NH3PbI3 Perovskite Solar Cells. Phys. Status Solidi A 2021, 218, 2100396. [Google Scholar] [CrossRef]
- Liu, C.; Yang, Y.; Rakstys, K.; Mahata, A.; Franckevicius, M.; Mosconi, E.; Skackauskaite, R.; Ding, B.; Brooks, K.G.; Usiobo, O.J.; et al. Tuning structural isomers of phenylenediammonium to afford efficient and stable perovskite solar cells and modules. Nat. Commun. 2021, 12, 6394. [Google Scholar] [CrossRef] [PubMed]
- Zardari, P.; Rostami, A.; Shekaari, H. p-Phenylenediaminium iodide capping agent enabled self-healing perovskite solar cell. Sci. Rep. 2020, 10, 20011. [Google Scholar] [CrossRef]
- Chen, Y.; Li, N.; Wang, L.; Li, L.; Xu, Z.; Jiao, H.; Liu, P.; Zhu, C.; Zai, H.; Sun, M.; et al. Impacts of alkaline on the defects property and crystallization kinetics in perovskite solar cells. Nat. Commun. 2019, 10, 1112. [Google Scholar] [CrossRef]
- Li, C.; Song, Z.; Chen, C.; Xiao, C.; Subedi, B.; Harvey, S.P.; Shrestha, N.; Subedi, K.K.; Chen, L.; Liu, D.; et al. Low-bandgap mixed tin–lead iodide perovskites with reduced methylammonium for simultaneous enhancement of solar cell efficiency and stability. Nat. Energy 2020, 5, 768–776. [Google Scholar] [CrossRef]
- Ueoka, N.; Oku, T.; Suzuki, A. Additive effects of alkali metals on Cu-modified CH3NH3PbI3−δClδ photovoltaic devices. RSC Adv. 2019, 9, 24231–24240. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, A.; Oku, T. Effects of transition metals incorporated into perovskite crystals on the electronic structures and magnetic properties by first-principles calculation. Heliyon 2018, 4, e00755. [Google Scholar] [CrossRef] [Green Version]
- Song, Z.; Xu, W.; Wu, Y.; Liu, S.; Bi, W.; Chen, X.; Song, H. Incorporating of Lanthanides Ions into Perovskite Film for Efficient and Stable Perovskite Solar Cells. Small 2020, 16, 2001770. [Google Scholar] [CrossRef]
- Suzuki, A.; Oku, T. Effects of mixed-valence states of Eu-doped FAPbI3 perovskite crystals studied by first-principles calculation. Mater. Adv. 2021, 2, 2609–2616. [Google Scholar] [CrossRef]
- Akman, E.; Shalan, A.E.; Sadegh, F.; Akin, S. Moisture-Resistant FAPbI3 Perovskite Solar Cell with 22.25% Power Conversion Efficiency through Pentafluorobenzyl Phosphonic Acid Passivation. ChemSusChem 2020, 14, 1176–1183. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Shen, Z.; Pan, L.; Han, J.; Eickemeyer, F.T.; Ren, Y.; Li, X.; Wang, S.; Liu, H.; Dong, X.; et al. Low-Cost Dopant Additive-Free Hole-Transporting Material for a Robust Perovskite Solar Cell with Efficiency Exceeding 21%. ACS Energy Lett. 2020, 6, 208–215. [Google Scholar] [CrossRef]
- Wan, L.; Zhang, W.; Fu, S.; Chen, L.; Wang, Y.; Xue, Z.; Tao, Y.; Zhang, W.; Song, W.; Fang, J. Achieving over 21% efficiency in inverted perovskite solar cells by fluorinating a dopant-free hole transporting material. J. Mater. Chem. A 2020, 8, 6517–6523. [Google Scholar] [CrossRef]
- Jeong, M.; Choi, I.W.; Go, E.M.; Cho, Y.; Kim, M.; Lee, B.; Jeong, S.; Jo, Y.; Choi, H.W.; Lee, J.; et al. Stable perovskite solar cells with efficiency exceeding 24.8% and 0.3-V voltage loss. Science 2020, 369, 1615–1620. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, M.; Suzuki, A.; Oku, T.; Ueoka, N.; Minami, S.; Okita, M. Effects of annealing temperature on decaphenylcyclopent silane-inserted CH3NH3PbI3 perovskite solar cells. Chem. Phys. Lett. 2019, 737, 136822. [Google Scholar] [CrossRef]
- Oku, T.; Kandori, S.; Taguchi, M.; Suzuki, A.; Okita, M.; Minami, S.; Fukunishi, S.; Tachikawa, T. Polysilane-Inserted Methylammonium Lead Iodide Perovskite Solar Cells Doped with Formamidinium and Potassium. Energies 2020, 13, 4776. [Google Scholar] [CrossRef]
- Oku, T.; Taguchi, M.; Suzuki, A.; Kitagawa, K.; Asakawa, Y.; Yoshida, S.; Okita, M.; Minami, S.; Fukunishi, S.; Tachikawa, T. Effects of Polysilane Addition to Chlorobenzene and High Temperature Annealing on CH3NH3PbI3 Perovskite Photovoltaic Devices. Coatings 2021, 11, 665. [Google Scholar] [CrossRef]
- Suzuki, A.; Taguchi, M.; Oku, T.; Okita, M.; Minami, S.; Fukunishi, S.; Tachikawa, T. Additive effects of methyl ammonium bromide or formamidinium bromide in methylammonium lead iodide perovskite solar cells using decaphenylcyclopentasilane. J. Mater. Sci. Mater. Electron. 2021, 32, 26449–26464. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, D.; Yu, Z.; Ma, W.; Li, H.-B.; Yang, X.; Liu, F.; Hagfeldt, A.; Sun, L. Molecular Engineering of Copper Phthalocyanines: A Strategy in Developing Dopant-Free Hole-Transporting Materials for Efficient and Ambient-Stable Perovskite Solar Cells. Adv. Energy Mater. 2018, 9, 1803287. [Google Scholar] [CrossRef]
- Matsuo, Y.; Ogumi, K.; Jeon, I.; Wang, H.; Nakagawa, T. Recent progress in porphyrin- and phthalocyanine-containing perovskite solar cells. RSC Adv. 2020, 10, 32678–32689. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, L.; Mu, X.; Chen, C.; Wu, Y.; Cao, J.; Tang, Y. Intramolecular Electric Field Construction in Metal Phthalocyanine as Dopant-Free Hole Transporting Material for Stable Perovskite Solar Cells with >21 % Efficiency. Angew. Chem. Int. Ed. 2021, 60, 6294–6299. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Hernández, A.; Kazim, S.; Ortiz, J.; Sastre-Santos, Á.; Ahmad, S. Molecularly engineered thienyl-triphenylamine substituted zinc phthalocyanine as dopant free hole transporting materials in perovskite solar cells. Sustain. Energy Fuels 2020, 4, 6188–6195. [Google Scholar] [CrossRef]
- Molina, D.; Ruiz-Preciado, M.A.; Carlsen, B.; Eickemeyer, F.T.; Yang, B.; Flores-Díaz, N.; Álvaro-Martins, M.J.; Nonomura, K.; Hagfeldt, A.; Sastre-Santos, Á. Zinc Phthalocyanine Conjugated Dimers as Efficient Dopant-Free Hole Transporting Materials in Perovskite Solar Cells. ChemPhotoChem 2020, 4, 307–314. [Google Scholar] [CrossRef]
- Javaid, S.; Lee, G. The impact of molecular orientation on carrier transfer characteristics at a phthalocyanine and halide perovskite interface. RSC Adv. 2021, 11, 31776–31782. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.-B.; Wang, L.-Y.; Mu, X.-J.; Zou, X.-X.; Wu, Y.-Y.; Cao, J. Lead and Iodide Fixation by Thiol Copper(II) Porphyrin for Stable and Environmental-Friendly Perovskite Solar Cells. CCS Chem. 2021, 3, 25–36. [Google Scholar] [CrossRef]
- Huang, P.; Hernández, A.; Kazim, S.; Follana-Berná, J.; Ortiz, J.; Lezama, L.; Sastre-Santos, Á.; Ahmad, S. Asymmetrically Substituted Phthalocyanines as Dopant-Free Hole Selective Layers for Reliability in Perovskite Solar Cells. ACS Appl. Energy Mater. 2021, 4, 10124–10135. [Google Scholar] [CrossRef]
- Hu, Q.; Rezaee, E.; Xu, W.; Ramachandran, R.; Chen, Q.; Xu, H.; Assaad, T.E.; McGrath, D.V.; Xu, Z.X. Dual defect-passivation using phthalocyanine for enhanced efficiency and stability of perovskite solar cells. Small 2021, 17, 2005216. [Google Scholar] [CrossRef]
- Esqueda, M.L.; Vergara, M.E.S.; Bada, J.R.; Salcedo, R. CuPc: Effects of its Doping and a Study of Its Organic-Semiconducting Properties for Application in Flexible Devices. Materials 2019, 12, 434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, A.; Kida, T.; Takagi, T.; Oku, T. Effects of hole-transporting layers of perovskite-based solar cells. Jpn. J. Appl. Phys. 2016, 55, 2BF01. [Google Scholar] [CrossRef]
- Suzuki, A.; Okumura, H.; Yamasaki, Y.; Oku, T. Fabrication and characterization of perovskite type solar cells using phthalocyanine complexes. Appl. Surf. Sci. 2019, 488, 586–592. [Google Scholar] [CrossRef]
- Suzuki, A.; Hayashi, Y.; Yamasaki, Y.; Oku, T. Fabrication and characterization of perovskite solar cells added with zinc phthalocyanine to active layer. AIP Conf. Proc. 2019, 2067, 20010. [Google Scholar] [CrossRef]
- Capitán, M.J.; Álvarez, J.; Navio, C. Study of the electronic structure of electron accepting cyano-films: TCNQversusTCNE. Phys. Chem. Chem. Phys. 2018, 20, 10450–10459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Devices | JSC | VOC | FF | RS | RSh | η | ηave |
|---|---|---|---|---|---|---|---|
| (mA cm−2) | (V) | (Ω cm2) | (Ω cm2) | (%) | (%) | ||
| CuPc(NH2)4-TCNQ | 8.39 | 0.716 | 0.626 | 6.90 | 5000 | 3.76 | 1.30 |
| CuPc(COOH)4-TCNQ | 16.8 | 0.655 | 0.491 | 3.14 | 152 | 5.49 | 4.45 |
| CuPc(SO3Na)4-TCNQ | 2.66 | 0.603 | 0.465 | 11.6 | 1160 | 0.75 | 0.54 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suzuki, A.; Hasegawa, R.; Oku, T.; Okita, M.; Fukunishi, S.; Tachikawa, T.; Hasegawa, T. Fabrication and Characterization of Perovskite Solar Cells Using Copper Phthalocyanine Complex with Tetracyanoquinodimethane. Chem. Proc. 2022, 9, 8. https://doi.org/10.3390/IOCC_2022-12154
Suzuki A, Hasegawa R, Oku T, Okita M, Fukunishi S, Tachikawa T, Hasegawa T. Fabrication and Characterization of Perovskite Solar Cells Using Copper Phthalocyanine Complex with Tetracyanoquinodimethane. Chemistry Proceedings. 2022; 9(1):8. https://doi.org/10.3390/IOCC_2022-12154
Chicago/Turabian StyleSuzuki, Atsushi, Ryota Hasegawa, Takeo Oku, Masanobu Okita, Sakiko Fukunishi, Tomoharu Tachikawa, and Tomoya Hasegawa. 2022. "Fabrication and Characterization of Perovskite Solar Cells Using Copper Phthalocyanine Complex with Tetracyanoquinodimethane" Chemistry Proceedings 9, no. 1: 8. https://doi.org/10.3390/IOCC_2022-12154
APA StyleSuzuki, A., Hasegawa, R., Oku, T., Okita, M., Fukunishi, S., Tachikawa, T., & Hasegawa, T. (2022). Fabrication and Characterization of Perovskite Solar Cells Using Copper Phthalocyanine Complex with Tetracyanoquinodimethane. Chemistry Proceedings, 9(1), 8. https://doi.org/10.3390/IOCC_2022-12154






