Abstract
The quaternary compounds AI2BIICIVX4, where AI–Cu, Ag; BII–Zn, Cd, Hg; CIV–Si, Ge, Sn; and X–S, Se, Te, crystallize in non-centrosymmetric structures and may be of interest for nonlinear optics. Here, we present in detail isothermal sections and physico-chemical equilibria in the Ag2Se–Zn(Cd, Hg, Pb)Se–SnSe2 systems where some of these compounds were found. The crystal structure of Ag2ZnSnSe4 was determined for the first time as the tetragonal symmetry, S.G. I2m, lattice parameters a = 0.60434(2), c = 1.13252(5) nm. No quaternary compounds were found in the Ag2Se–PbSe–SnSe2 system. Ag8SnSe6–PbSe is the triangulating section in this system.
1. Introduction
The formation of quaternary compounds in the AI2X–BIIX–CIVX2 systems where AI–Cu, Ag; BII–Zn, Cd, Hg; CIV–Si, Ge, Sn; and X–S, Se, Te is known for seven component combinations [1]. The most common are the phases with the equimolar ratio of all three binary compounds described by the AI2BIICIVX4 formula. These quaternary compounds crystallize in non-centrosymmetric structures and may be of interest for nonlinear optics. Ag-containing compounds may be of interest due to the possible formation of compounds with high ionic conductivity [2,3].
The boundary sides of the presented systems Ag2Se–Zn(Cd, Hg, Pb)Se–SnSe2 feature only two compounds, Ag8SnSe6 (Ag2Se–SnSe2 system) and Hg2SnSe4 (HgSe–SnSe2 system). High-temperature modification of Ag8SnSe6 crystallizes in fcc structure (S.G. P4232); the crystal structure of the low-temperature Ag8SnSe6 was investigated using X-ray powder diffraction. This modification crystallizes in the orthorhombic unit cell (S.G. Pmn21) and is isostructural to β’-Ag8GeSe6. Hg2SnSe4 crystallizes in the thiogallate structure (defect chalcopyrite, S.G I).
The Ag2Se–ZnSe–SnSe2 and Ag2Se–CdSe–SnSe2 systems contain only one intermediate quaternary compound each, Ag2ZnSnSe4 and Ag2CdSnSe4 [4]. Each compound has at 670 K a minor homogeneity region stretched along the Ag33.3Sn16.7Se50–Zn(Cd)Se sections. Due to the absence of a ternary compound, the sections are non-quasi-binary in the range of 0–50 mol.% Zn(Cd)Se. The crystal structure of the Ag2CdSnSe4 compound was determined in the orthorhombic symmetry, S.G. Cmc21, a = 0.42640(2), b = 0.73170(3), c = 0.69842(4) nm, RI = 0.0782) [4]. The Ag8SnSe6–Zn(Cd)Se sections of these systems are quasi-binary, of the eutectic type, with large solid solution ranges of end compounds [5].
The Ag2Se–HgSe–SnSe2 system [6,7,8] features at 670 K three intermediate phases, Ag2HgSnSe4, Ag4Hg3Sn2Se9 (Ag2.66Hg2Sn1.34Se6) and Ag6HgSnSe6. Ag2HgSnSe4 crystallizes in the orthorhombic S.G. Pmn21, with lattice periods a = 0.8461(1), b = 0.7340(1) and c = 0.69901(6) nm [6,8]. The Ag4Hg3Sn2Se9 compound crystallizes in an orthorhombic unit cell (S.G. Imm2, a = 1.2795(2), b = 0.42631(6) and c = 0.58207(4) nm) [7]. This compound has a homogeneity region that is stretched to the ternary compound Hg2SnSe4 (the Ag2Se content is 15–28 mol.%) and is negligible along the Ag33.3Sn16.7Se50–HgSe section. The unit cell periods decrease within the homogeneity region to a = 1.2665(3), b = 0.4222(1) and c = 0.5739(1) nm. The structure of Ag6HgSnSe6 has not been investigated.
2. Materials and Methods
The alloys for investigation were prepared from high purity elements and the previously synthesized mercury selenide. The alloys were synthesized in evacuated quartz containers placed in a shaft-type furnace. The ampoules were heated to 1100 K at the rate of 50 K/h, kept for 6 h, then cooled at the rate of 10 K/h to 670 K. The alloys were annealed at this temperature for 500 h followed by quenching in air. Obtained ingots were compact and black.
The alloys were studied by differential thermal analysis (computer-controlled set-up of Thermodent T-04 furnace, Pt/Pt-Rh thermocouple) and powder X-ray diffraction (DRON 4-13 diffractometer, CuKα radiation).
3. Results and Discussion
3.1. Phase Equilibria in the Ag2Se–PbSe–SnSe2 System
Isothermal sections at room temperature of the title systems Ag2Se–Zn(Cd, Hg, Pb)–SnSe2 are presented in Figure 1. The systems with BII–Zn, Cd and Hg were discussed in in the introduction and are shown here for visual comparison.
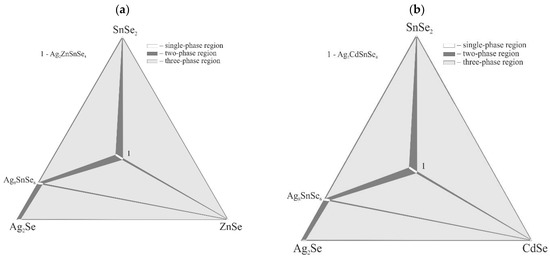
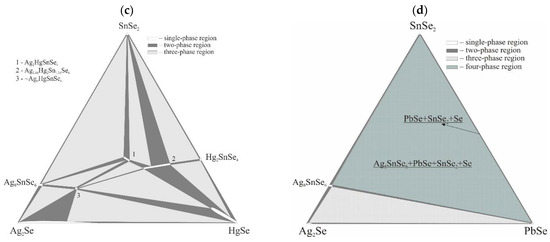
Figure 1.
Isothermal sections of the Ag2Se–Zn(Cd, Hg, Pb)Se–SnSe2 systems at room temperature ((a)—ZnSe, (b)—CdSe, (c)—HgSe, (d)—PbSe).
No quaternary compounds were found in the Ag2Se–PbSe–SnSe2 system. The alloys in the Ag8SnSe6–PbSe–SnSe2 sub-system are four-phase since the PbSe–SnSe2 section is non-quasi-binary [9]. Thus, the isothermal section consists of two three-phase fields, Ag2Se + Ag8SnSe6 + PbSe and PbSe + SnSe2 + Se (along the PbSe–SnSe2 line), one four-phase field Ag8SnSe6 + PbSe + SnSe2 + Se and contains four two-phase equilibria.
Ag8SnSe6–PbSe is the only triangulating section in this system (Figure 2). The section is quasi-binary, features a eutectic at 885 K and 67 mol.% PbSe and is quite similar to the previously referenced Ag8SnSe6–Zn(Cd)Se sections [5].
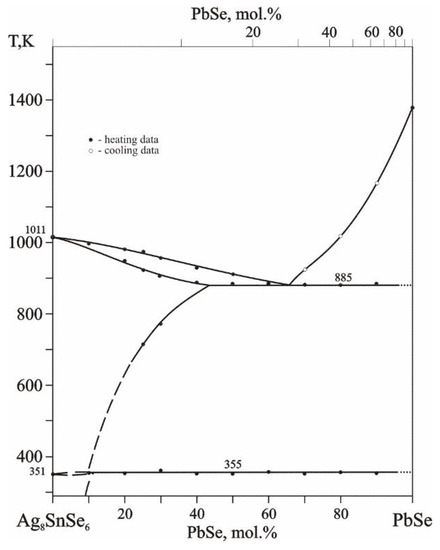
Figure 2.
Phase diagram of the Ag8SnSe6–PbSe section (top scale is PbSe content within the Ag2Se–PbSe–SnSe2 system).
3.2. Crystal Structure of the Quaternary Compound Ag2ZnSnSe4
The crystal structure of the Ag2ZnSnSe4 compound was determined by X-ray powder method. The set of the experimental intensities of diffraction reflections was recorded in the 2Θ range 10–100° with scan step 0.05° and 20 s exposure in each point at a DRON 4-13 diffractometer (CuKα radiation). The diffraction pattern of the obtained compound was indexed well in the tetragonal structure of the Cu2FeSnS4 stannite type with the parameters listed in Table 1. The refinement of profile and structure parameters of Ag2ZnSnSe4 in isotropic approximation yielded in the selected model the fit factors RI = 0.0570 and RP = 0.1277.

Table 1.
Results of the crystal structure determination of the Ag2ZnSnSe4 compound.
Experimental and theoretical X-ray diffraction patterns of the Ag2ZnSnSe4 compound and their differences are plotted in Figure 3. Atomic coordinates, site occupation and isotropic parameters of temperature displacement of the atoms in the structure of this quaternary chalcogenide are listed in Table 2. According to the obtained results, the structure formula of the quaternary compound is identical to the stoichiometric Ag2ZnSnSe4.
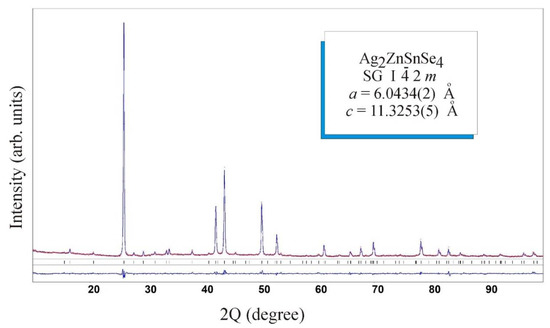
Figure 3.
Experimental and theoretical X-ray diffraction patterns for Ag2ZnSnSe4 and their differences.

Table 2.
Atomic coordinates and isotropic temperature displacement factors for the Ag2ZnSnSe4 structure.
The location of the atoms in the unit cell, coordination surrounding and the interatomic distances in the structure of the investigated compound are shown in Figure 4. All atoms are characterized by tetrahedral surrounding. Interatomic distances in the quaternary compound are consistent with the sum of the effective ionic radii.
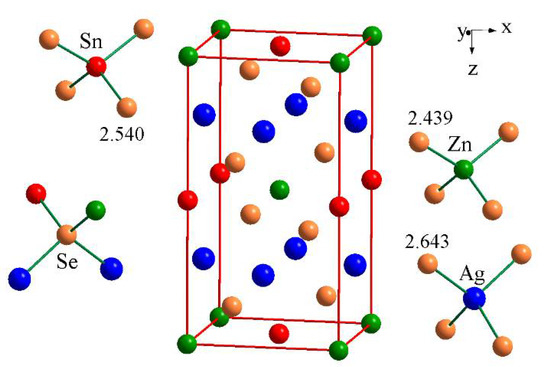
Figure 4.
Location of atoms in the unit cell, coordination surrounding and interatomic distances in the Ag2ZnSnSe4 structure.
The second coordination surrounding (SCS) [10] of selenium atoms shown in Figure 5 has the shape of a cuboctahedron within which the atoms of metallic components occupy four tetrahedral cavities. Comparing the crystal structure of Ag2ZnSnSe4 and the components and compounds of the Ag2Se–ZnSe–SnSe2 system, it should be noted that in terms of SCS and its content, the Ag2ZnSnSe4 compound is related to the sphalerite structure of room-temperature ZnSe [11]. Therefore, the crystal structure of Ag2ZnSnSe4 can be derived from the cubic sphalerite structure by doubling the unit cell along the c axis and ordering the sites of the atoms of the metallic components. SCS of selenium atoms in the structure of Ag0.67Sn0.33Se [12] is also of the sphalerite type where the atoms of the statistical mixture of cations occupy octahedral cavities within the SCS. Conversely, in the binary tin selenide SnSe2 [13], the wurtzite-type SCS is in the form of the hexagonal analog of a cuboctahedron, where tin atoms also occupy octahedral cavities. As for the binary silver selenide Ag2Se at room temperature [14], Ag1 atoms occupy tetrahedral voids and Ag2 atoms occupy octahedral voids within the wurtzite-type SCS.
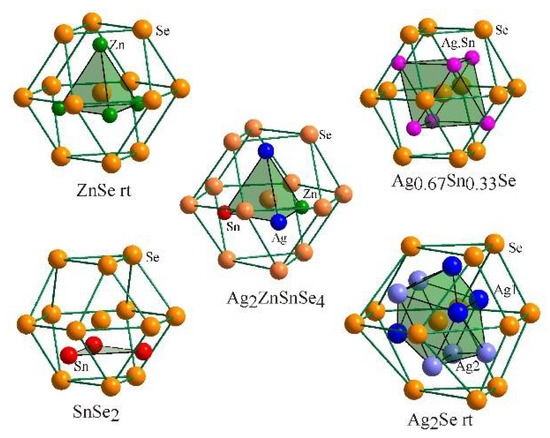
Figure 5.
Second coordination surrounding of selenium atoms in the structure of Ag2ZnSnSe4 and related selenides.
Thus, in the quaternary compound, only zinc atoms occupy the same sites as in the binary selenide, whereas silver and tin atoms occupy atypical octahedral voids within an atypical SCS, which is abnormal for them and can produce interesting physical properties in materials based on Ag2ZnSnSe4.
Supplementary Materials
The poster presentation can be downloaded at: https://www.mdpi.com/article/10.3390/IOCC_2022-12155/s1.
Author Contributions
Conceptualization, L.P., O.P.; methodology, I.O., O.P.; investigation, O.V., Y.K., L.P.; formal analysis, O.V., Y.K., L.P. (phase equilibria), A.F. (crystal structure); validation, Y.K., L.P.; data curation, A.F., Y.K., L.P.; writing—original draft preparation, O.V., Y.K., L.P.; writing—review and editing, Y.K., L.P., A.F., I.O., O.P.; visualization, O.V., Y.K., L.P. (phase equilibria), A.F. (crystal structure); supervision, O.P.; project administration, I.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Parasyuk, O.V.; Piskach, L.V.; Romanyuk, Y.E.; Olekseyuk, I.D.; Zaremba, V.I.; Pekhnyo, V.I. Phase relations in the quasi-binary Cu2GeS3–ZnS and quasi-ternary Cu2S–Zn(Cd)S–GeS2 systems and crystal structure of Cu2ZnGeS4. J. Alloys Compd. 2005, 397, 85–94. [Google Scholar] [CrossRef]
- Parasyuk, O.V.; Gulay, L.D.; Romanyuk, Y.E.; Olekseyuk, I.D. The Ag2Se–HgSe–SiSe2 system in the 0-60 mol. % SiSe2 region. J. Alloys Compd. 2003, 348, 157–166. [Google Scholar] [CrossRef]
- Parasyuk, O.V.; Gulay, L.D.; Romanyuk, Y.E.; Olekseyuk, I.D.; Piskach, L.V. The Ag2Se–HgSe–GeSe2 system and crystal structures of the compounds. J. Alloys Compd. 2003, 351, 135–144. [Google Scholar] [CrossRef]
- Parasyuk, O.V.; Gulay, L.D.; Piskach, L.V.; Olekseyuk, I.D. The Ag2Se–CdSe–SnSe2 system at 670 K and the crystal structure of the Ag2CdSnSe4 compd. J. Alloys Compd. 2002, 335, 176–180. [Google Scholar] [CrossRef]
- Piskach, L.V.; Parasyuk, O.V.; Olekseyuk, I.D.; Romanyuk, Y.E.; Volkov, S.V.; Pekhnyo, V.I. Interaction of argyrodite family compounds with the chalcogenides of II-b elements. J. Alloys Compd. 2006, 421, 98–104. [Google Scholar] [CrossRef]
- Parasyuk, O.V. Phase relations of the Ag2SnS3–HgS and Ag33.3Sn16.7Se(Te)50–HgSe(Te) section in Ag–Hg–Sn–S(Se,Te) systems. J. Alloys Compd. 1999, 291, 215–219. [Google Scholar] [CrossRef]
- Parasyuk, O.V.; Gulay, L.D. Crystal structure of the Ag2.66Hg2Sn1.34Se6 and Hg2SnSe4 compounds. J. Alloys Compd. 2002, 337, 94–98. [Google Scholar]
- Parasyuk, O.V.; Gulay, L.D.; Piskach, L.V.; Kumanska, Y.O. The Ag2Se—HgSe—SnSe2 System and the Crystal Structure of the Ag2HgSnSe4 Compound. J. Alloys Compd. 2002, 339, 140–143. [Google Scholar] [CrossRef]
- Dal Corso, S.; Liautard, B.; Tedenac, J.C. The Pb–Sn–Se System: Phase Equilibria and Reactions in the PbSe–SnSe–Se SubTernary. J. Phase Equilibria. 1995, 16, 308–314. [Google Scholar] [CrossRef]
- Fedorchuk, A.O.; Parasyuk, O.V.; Kityk, I.V. Second anion coordination for wurtzite and sphalerite chalcogenide derivatives as a tool for the description of anion sub-lattice. Mater. Chem. Phys. 2013, 139, 92–99. [Google Scholar] [CrossRef]
- Wagner, G.; Lehmann, S.; Schorr, S.; Spemann, D.; Doering, T. The two-phase region in 2(ZnSe)x(CuInSe2)1−x alloys and structural relation between the tetragonal and cubic phases. J. Solid State Chem. 2005, 178, 3631–3638. [Google Scholar] [CrossRef]
- Wold, A.; Brec, R. Structure NaCl des phases AgxSn1−xX (X = S, Se). Mater. Res. Bull. 1976, 11, 761–765. [Google Scholar] [CrossRef]
- Liu, H.; Chang, L.L.Y. Phase relations in systems of tin chalcogenides. J. Alloys Compd. 1992, 185, 183–190. [Google Scholar] [CrossRef]
- Billetter, H.; Ruschewitz, U. Structural phase transitions in Ag2Se (naumannite). Z. Anorg. Allg. Chem. 2008, 634, 241–246. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).