X-ray Diffraction Study of Fluorine-Functionalized Thiosemicarbazones and Cyclometallated Compounds †
Abstract
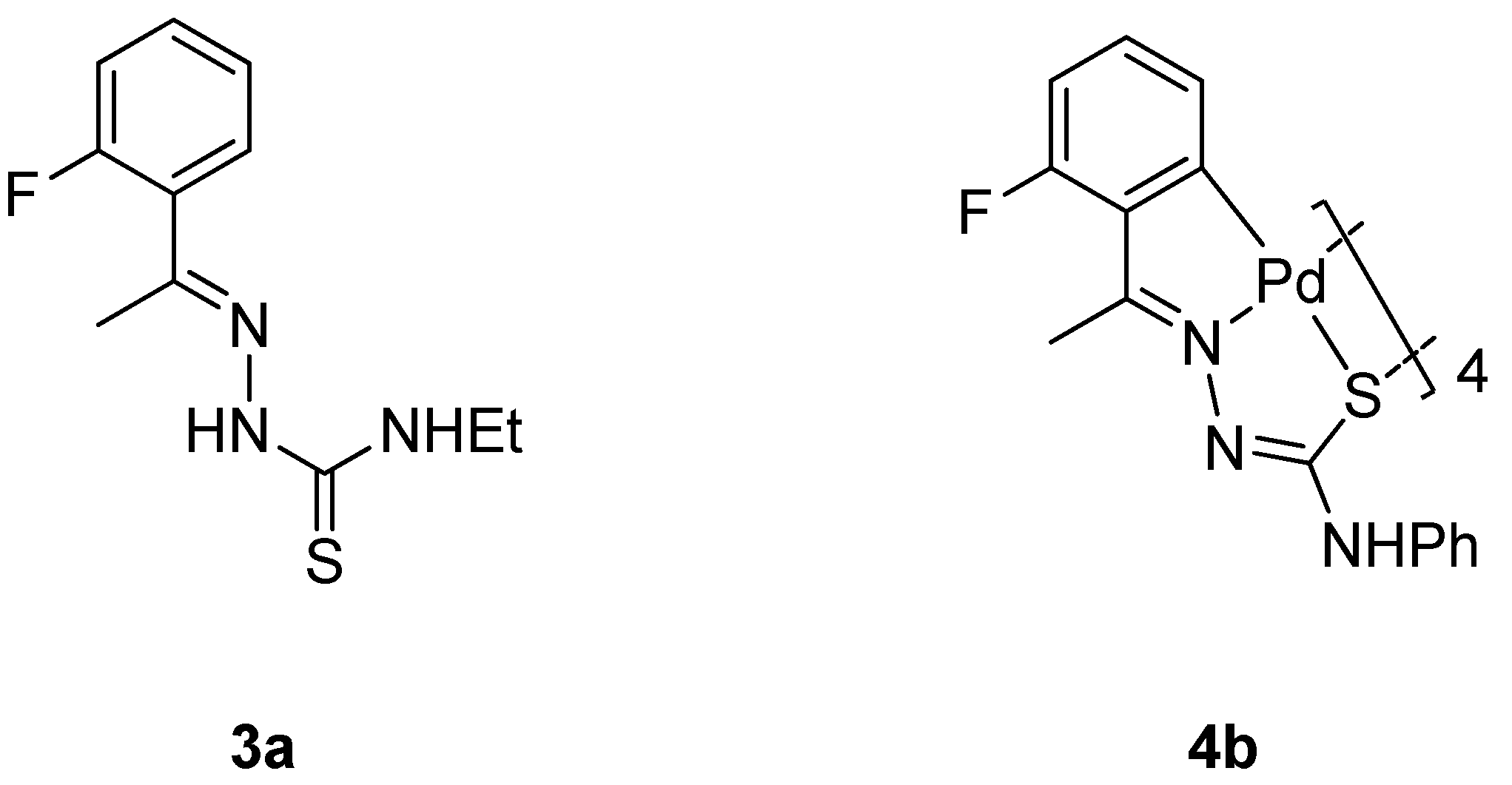
:1. Introduction
2. Results and Discussion
2.1. X-ray Diffraction Study
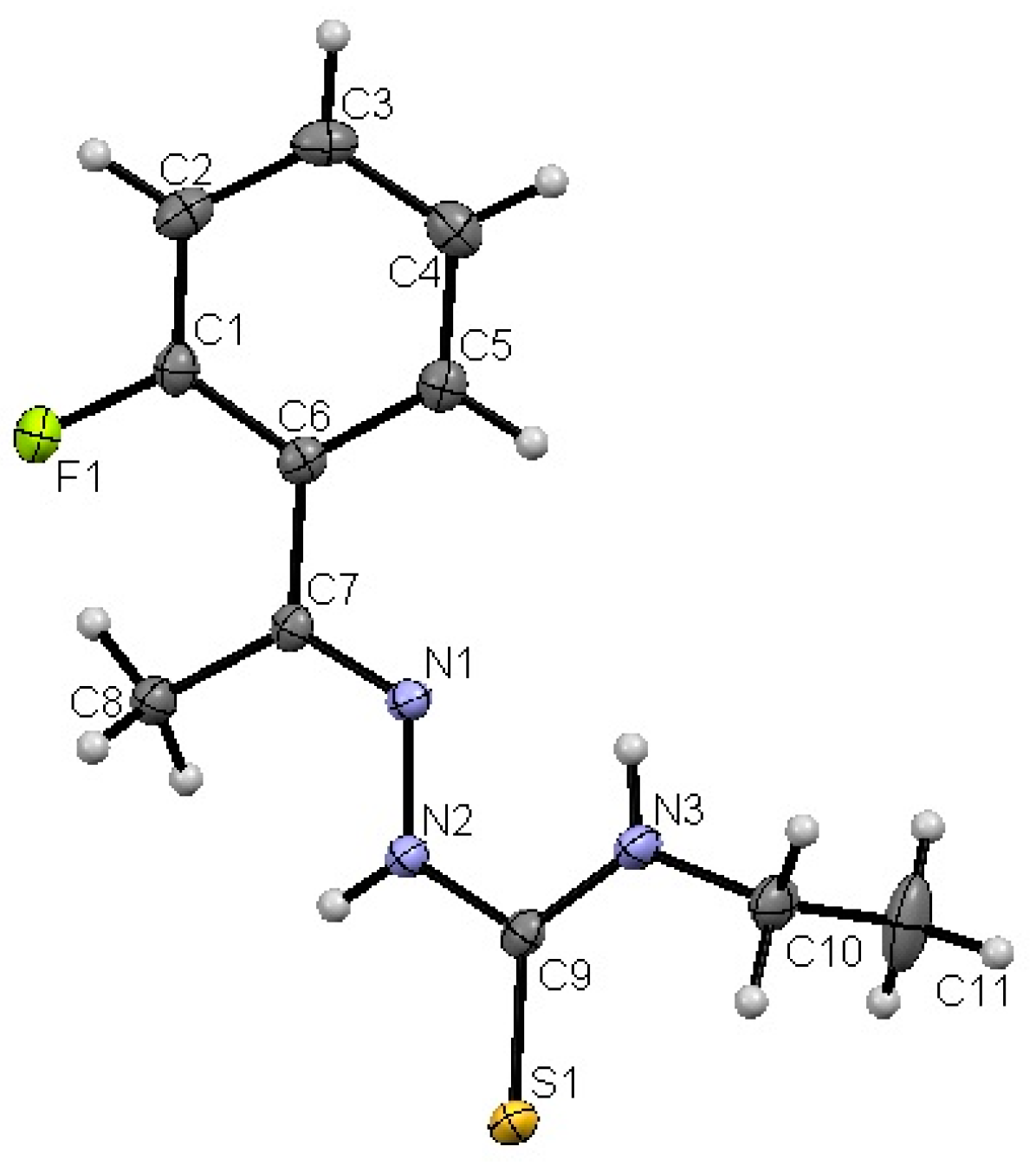
2.1.1. Compound 3a
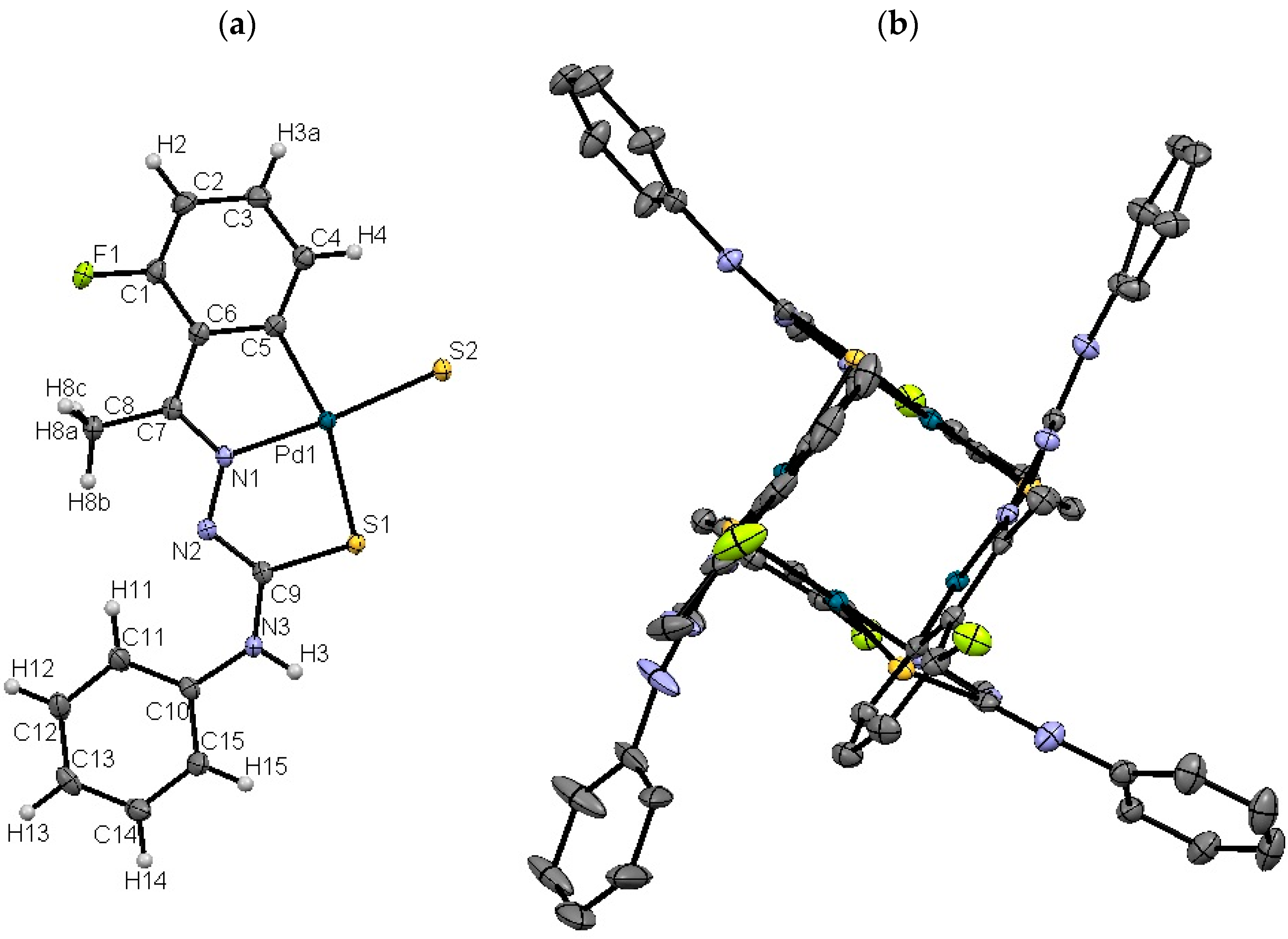
2.1.2. Compound 4b
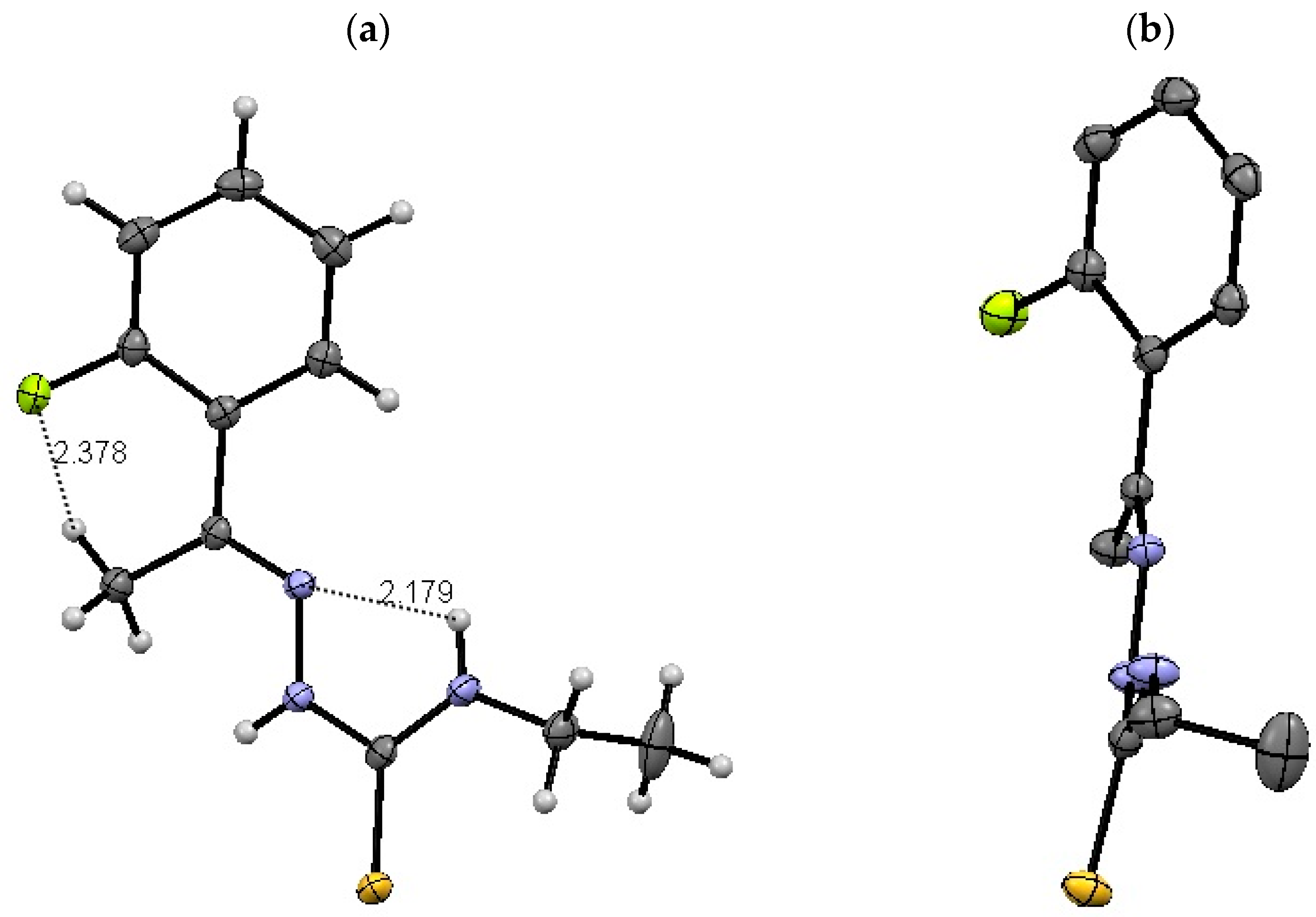
2.1.3. Comparison between Bond Distances (Å) and Angles (°)
2.1.4. Palladium Bonds and Angles in Compound 4b
3. Conclusions
- X-ray structural analysis was carried out for a thiosemicarbazone ligand and its cyclometallated palladium derivative.
- A comparative study allowed the determination of variations in bond distances and angles in the structure of the ligand after the cyclometallation process.
- The metal atom displays the typical square-planar geometry for palladium.
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Identification code | 3a |
| Empirical formula | C11H14FN3S |
| Formula weight | 239.31 |
| Temperature | 100(2) K |
| Wavelength | 0.71073 Å |
| Crystal system | Monoclinic |
| Space group | P21/n |
| Unit cell dimensions | a = 5.8663(2) Å, α = 90° |
| b = 19.5573(6) Å, β = 105.0047(10)°. | |
| c = 10.5485(3) Å, γ = 90°. | |
| Volume | 1168.96(6) Å3 |
| Z | 4 |
| Density (calculated) | 1.360 Mg/m3 |
| Absorption coefficient | 0.266 mm−1 |
| F(000) | 504 |
| Crystal size | 0.240 × 0.127 × 0.119 mm3 |
| Theta range for data collection | 2.083 to 26.366°. |
| Index ranges | −6 ≤ h ≤ 7, −24 ≤ k ≤ 24, −13 ≤ l ≤ 13 |
| Reflections collected | 32894 |
| Independent reflections | 2399 [R(int) = 0.0527] |
| Completeness to theta = 25.242° | 100.0% |
| Refinement method | Full-matrix least-squares on F2 |
| Data/restraints/parameters | 2399/0/147 |
| Goodness-of-fit on F2 | 1.050 |
| Final R indices [I > 2sigma(I)] | R1 = 0.0302, wR2 = 0.0712 |
| R indices (all data) | R1 = 0.0372, wR2 = 0.0751 |
| Largest diff. peak and hole | 0.246 and −0.266 e·Å−3 |
| Identification code | 4b |
| Empirical formula | C64H52Cl12F4N12Pd4S4 |
| Formula weight | 2044.41 |
| Temperature | 100(2) K |
| Wavelength | 0.71073 Å |
| Crystal system | Triclinic |
| Space group | P-1 |
| Unit cell dimensions | a = 13.6814(4) Å, α = 87.1620(10)° |
| b = 15.1512(4) Å, β = 79.4790(10)° | |
| c = 19.9092(5) Å, γ = 64.1110(10)° | |
| Volume | 3648.25(17) Å3 |
| Z | 2 |
| Density (calculated) | 1.861 Mg/m3 |
| Absorption coefficient | 1.585 mm−1 |
| F(000) | 2016 |
| Crystal size | 0.180 × 0.160 × 0.070 mm3 |
| Theta range for data collection | 2.082 to 28.342°. |
| Index ranges | −18 ≤ h ≤ 18, −20 ≤ k ≤ 20, −26 ≤ l ≤ 26 |
| Reflections collected | 112138 |
| Independent reflections | 18195 [R(int) = 0.0390] |
| Completeness to theta = 25.242° | 99.9% |
| Refinement method | Full-matrix least-squares on F2 |
| Data/restraints/parameters | 18195/0/1020 |
| Goodness-of-fit on F2 | 1.049 |
| Final R indices [I>2sigma(I)] | R1 = 0.0299, wR2 = 0.0625 |
| R indices (all data) | R1 = 0.0390, wR2 = 0.0666 |
| Largest diff. peak and hole | 2.352 and −1.613 e·Å−3 |
References
- Abás, E.; Gómez-Bachiller, M.; Colom, E.; Pardina, E.; Rodríguez-Diéguez, A.; Grasa, L.; Laguna, M. Cyclometallated gold(III) complexes against colon cancer. X-ray structure of [Au(C,NPhenylpyridine)(OAc)2]. J. Organomet. Chem. 2020, 920, 121340. [Google Scholar] [CrossRef]
- Tong, X.; Zhang, L.; Li, L.; Li, Y.; Yang, Z.; Zhu, D.; Xie, Z. Water-soluble cyclometalated Ir (III) complexes as carrier-free and pure nanoparticle photosensitizers for photodynamic therapy and cell imaging. Dalton Trans. 2020, 49, 11493–11497. [Google Scholar] [CrossRef] [PubMed]
- Lobana, T.S.; Sharma, R.; Bawa, G.; Khanna, S. Bonding and structure trends of thiosemicarbazone derivatives of metals—An overview. Coord. Chem. Rev. 2009, 253, 977–1055. [Google Scholar] [CrossRef]
- Srishailam, K.; Ramaiah, K.; Reddy, K.L.; Reddy, B.V.; Rao, G.R. Synthesis and evaluation of molecular structure from torsional scans, study of molecular characteristics using spectroscopic and dft methods of some thiosemicarbazones, and investigation of their anticancer activity. Chem. Pap. 2021, 75, 3635–3647. [Google Scholar] [CrossRef]
- Mrozek-Wilczkiewicz, A.; Malarz, K.; Rejmund, M.; Polanski, J.; Musiol, R. Anticancer activity of the thiosemicarbazones that are based on di-2-pyridine ketone and quinoline moiety. Eur. J. Med. Chem. 2019, 171, 180–194. [Google Scholar] [CrossRef]
- Yousef, T.A.; Abu El-Reash, G.M. Synthesis, and biological evaluation of complexes based on thiosemicarbazone ligand. J. Mol. Struct. 2020, 1201, 127180. [Google Scholar] [CrossRef]
- Özerkan, D.; Ertik, O.; Kaya, B.; Kuruca, S.E.; Yanardag, R.; Ülküseven, B. Novel palladium (II) complexes with tetradentate thiosemicarbazones. Synthesis, characterization, in vitro cytotoxicity and xanthine oxidase inhibition. Investig. New Drugs 2019, 37, 1187–1197. [Google Scholar] [CrossRef]
- Zaera, F. An organometallic guide to the chemistry of hydrocarbon moieties on transition metal surfaces. Chem. Rev. 1995, 95, 2651–2693. [Google Scholar] [CrossRef]
- Jain, V.K. Cyclometalated group-16 compounds of palladium and platinum: Challenges and opportunities. Coord. Chem. Rev. 2021, 427, 213546. [Google Scholar] [CrossRef]
- Munín-Cruz, P.; Reigosa, F.; Rúa-Sueiro, M.; Ortigueira, J.M.; Pereira, M.T.; Vila, J.M. Chemistry of tetradentate [C,N : C,N] iminophosphorane palladacycles: Preparation, reactivity and theoretical calculations. ChemistryOpen 2020, 9, 1190–1194. [Google Scholar] [CrossRef]
- Lucio-Martínez, F.; Bermúdez, B.; Ortigueira, J.M.; Adams, H.; Fernández, A.; Pereira, M.T.; Vila, J.M. A highly effective strategy for encapsulating potassium cations in small crown ether rings on a dinuclear palladium complex. Chem.—Eur. J. 2017, 23, 6255–6258. [Google Scholar] [CrossRef] [PubMed]
- Meng, K.; Li, T.; Yu, C.; Shen, C.; Zhang, J.; Zhong, G. Geminal group-directed olefinic CH functionalization via four-to eight-membered exo-metallocycles. Nat. Commun. 2019, 10, 5109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.-J.; Ziegler, M.S.; Tilley, T.D. Synthesis, structures, and reactivity studies of cyclometalated N-heterocyclic carbene complexes of ruthenium. Dalton Trans. 2018, 47, 12138–12146. [Google Scholar] [CrossRef] [PubMed]
- Samiee, S.; Shiralinia, A.; Hoveizi, E.; Gable, R.W. A new family of oxime palladacycles mixed with unsymmetrical phosphorus ylides; synthesis, structural, cytotoxicity and catalytic activity studies. J. Organomet. Chem. 2019, 900, 120927. [Google Scholar] [CrossRef]
- Dharani, S.; Kalaiarasi, G.; Sindhuja, D.; Lynch, V.M.; Shankar, R.; Karvembu, R.; Prabhakaran, R. Tetranuclear palladacycles of 3-Acetyl-7-methoxy-2H-chromen-2-one derived Schiff Bases: Efficient catalysts for Suzuki–Miyaura coupling in an aqueous medium. Inorg. Chem. 2019, 58, 8045–8055. [Google Scholar] [CrossRef]
- López-Mosquera, C.; Grabulosa, A.; Rocamora, M.; Font-Bardia, M.; Muller, G. Cyclopalladated compounds with polyhalogenated benzylphosphanes for the Mizoroki-Heck reaction. Eur. J. Inorg. Chem. 2020, 2020, 2470–2484. [Google Scholar] [CrossRef]
- Maji, A.; Singh, O.; Singh, S.; Mohanty, A.; Maji, P.K.; Ghosh, K. Palladium-based catalysts supported by unsymmetrical XYC–1 type pincer ligands: C5 arylation of imidazoles and synthesis of octinoxate utilizing the Mizoroki–Heck reaction. Eur. J. Inorg. Chem. 2020, 2020, 1596–1611. [Google Scholar] [CrossRef]
- Serrano, J.L.; Girase, T.R. Palladacycles as efficient precatalysts for Negishi and Buchwald-Hartwig amination reactions. In Palladacycles; Elsevier: Amsterdam, The Netherlands, 2019; pp. 175–224. [Google Scholar]
- Yu, F.; Shen, W.; Sun, Y.; Liao, Y.; Jin, S.; Lu, X.; He, R.; Zhong, L.; Zhong, G.; Zhang, J. Ruthenium-catalyzed C-H amination of aroylsilanes. Org. Biomol. Chem. 2021, 19, 6313–6321. [Google Scholar] [CrossRef]
- Pike, S.; Lord, R.; Kergreis, A. Influence of ligand and nuclearity on the cytotoxicity of cyclometallated C^N^C platinum (II) complexes. Chem.—Eur. J. 2020, 26, 14938–14946. [Google Scholar]
- Oliveira, C.G.; Romero-Canelón, I.; Coverdale, J.P.; Maia, P.I.S.; Clarkson, G.J.; Deflon, V.M.; Sadler, P.J. Novel tetranuclear Pd II and Pt II anticancer complexes derived from pyrene thiosemicarbazones. Dalton Trans. 2020, 49, 9595–9604. [Google Scholar] [CrossRef]
- Licona, C.; Delhorme, J.-B.; Riegel, G.; Vidimar, V.; Cerón-Camacho, R.; Boff, B.; Venkatasamy, A.; Tomasetto, C.; Gomes, P.d.S.F.C.; Rognan, D. Anticancer activity of ruthenium and osmium cyclometalated compounds: Identification of ABCB1 and EGFR as resistance mechanisms. Inorg. Chem. Front. 2020, 7, 678–688. [Google Scholar] [CrossRef]
- Williams, M.R.; Bertrand, B.; Hughes, D.L.; Waller, Z.A.; Schmidt, C.; Ott, I.; O’Connell, M.; Searcey, M.; Bochmann, M. Cyclometallated Au (III) dithiocarbamate complexes: Synthesis, anticancer evaluation and mechanistic studies. Metallomics 2018, 10, 1655–1666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rúa Sueiro, M.; Ortigueira, J.M.; Vila, J.M. Cyclometallated thiosemicarbazones containing fluorine atoms: A solubility improvement. In Proceedings of the 25th International Electronic Conference on Synthetic Organic Chemistry; MDPI: Basel, Switzerland, 2021. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.; Puschmann, H. Olex2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Casas, J.; Garcıa-Tasende, M.; Sordo, J. Main group metal complexes of semicarbazones and thiosemicarbazones. A structural review. Coord. Chem. Rev. 2000, 209, 197–261. [Google Scholar] [CrossRef]
- Rúa-Sueiro, M.; Munín-Cruz, P.; Reigosa, F.; Vila, J.M.; Ortigueira, J.M. Synthesis and X-Ray diffraction study of thiosemicarbazone palladacycles with dppm. Proceedings 2020, 62, 13. [Google Scholar]
- Martínez, J.; Cabaleiro-Lago, E.M.; Ortigueira, J.M.; Pereira, M.T.; Frieiro, P.; Lucio, F.; Vila, J.M. Synthesis and reactivity of thiosemicarbazone palladacycles. Crystal structure analysis and theoretical calculations. Inorg. Chim. Acta 2016, 449, 20–30. [Google Scholar] [CrossRef]
- Pereira, M.T.; Antelo, J.M.; Adrio, L.A.; Martinez, J.; Ortigueira, J.M.; Lopez-Torres, M.; Vila, J.M. Novel bidentate [N,S] palladacycle metalloligands. 1H-15N HMBC as a decisive NMR technique for the structural characterization of Palladium-Rhodium and Palladium-Palladium bimetallic complexes. Organometallics 2014, 33, 3265–3274. [Google Scholar] [CrossRef]

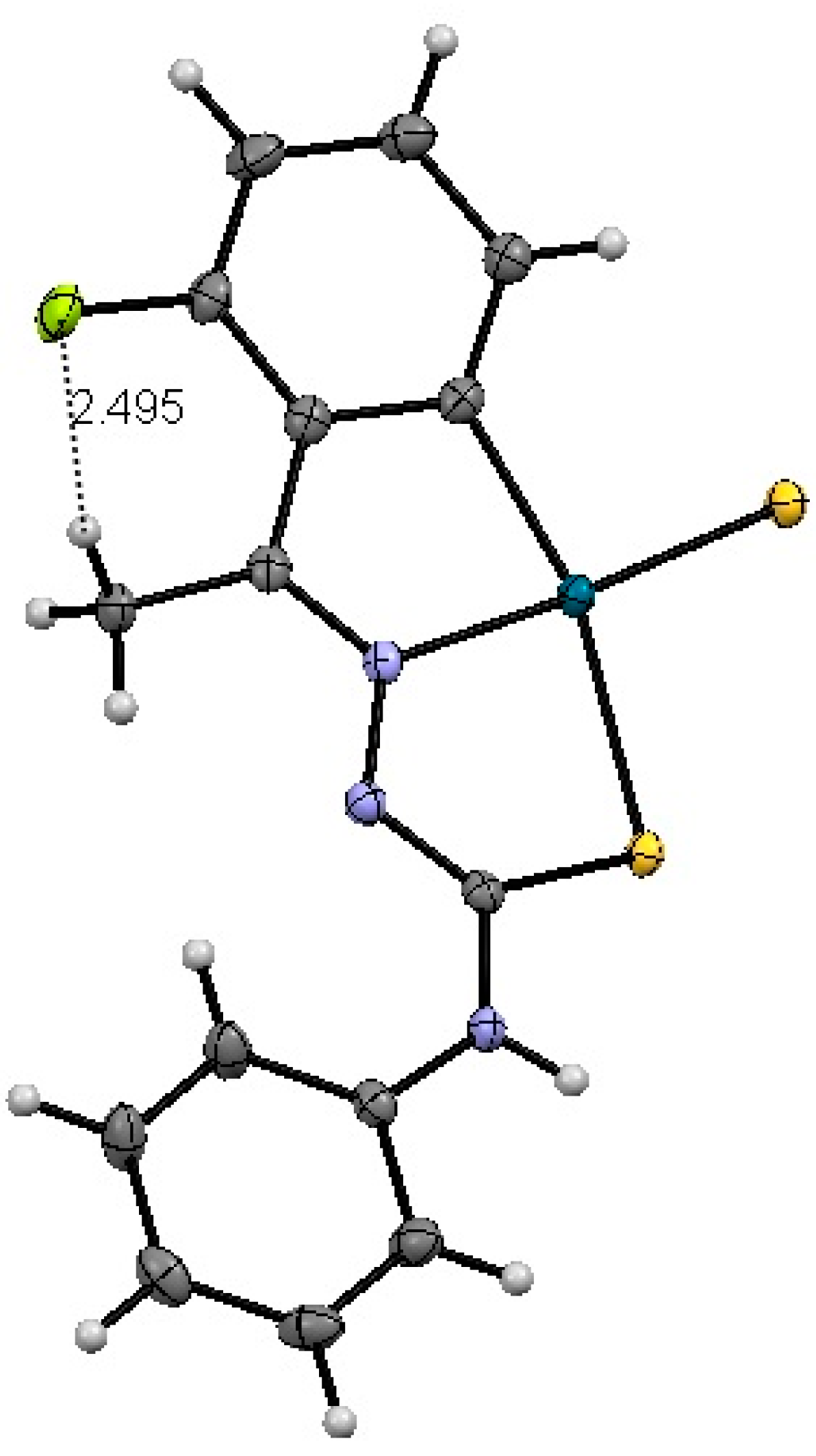
| Bond | 3a/Å | 4b/Å |
|---|---|---|
| N(1)-C(7) | 1.2870(18) | 1.304(3) |
| C(9)-N(2) | 1.3677(18) | 1.297(3) |
| C(9)-S(1) | 1.6816(14) | 1.807(3) |
| C(9)-N(3) | 1.3297(18) | 1.361(3) |
| C(5)-C(6) | 1.399(2) | 1.424(4) |
| Angle | 3a/° | 4b/° |
|---|---|---|
| C(5)-C(6)-C(7) | 121.14(12) | 116.3(2) |
| N(1)-N(2)-C(9) | 117.90(11) | 114.1(2) |
| N(2)-C(9)-S(1) | 119.57(11) | 125.16(19) |
| N(2)-C(9)-N(3) | 115.62(12) | 120.6(2) |
| N(3)-C(9)-S(1) | 124.81(11) | 114.08(18) |
| Bond | /Å | Angle | /° |
|---|---|---|---|
| Pd(1)-N(1) | 1.996(2) | N(1)-Pd(1)-C(5) | 81.24(9) |
| Pd(1)-C(5) | 2.003(2) | N(1)-Pd(1)-S(1) | 83.31(6) |
| Pd(1)-S(2) | 2.3060(6) | C(5)-Pd(1)-S(2) | 94.39(8) |
| Pd(1)-S(1) | 2.3729(6) | S(2)-Pd(1)-S(1) | 100.81(2) |
| N(1)-Pd(1)-S(2) | 174.68(6) | ||
| C(5)-Pd(1)-S(1) | 164.07(8) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rúa-Sueiro, M.; Munín-Cruz, P.; Ortigueira, J.M.; Vila, J.M. X-ray Diffraction Study of Fluorine-Functionalized Thiosemicarbazones and Cyclometallated Compounds. Chem. Proc. 2022, 9, 3. https://doi.org/10.3390/IOCC_2022-12140
Rúa-Sueiro M, Munín-Cruz P, Ortigueira JM, Vila JM. X-ray Diffraction Study of Fluorine-Functionalized Thiosemicarbazones and Cyclometallated Compounds. Chemistry Proceedings. 2022; 9(1):3. https://doi.org/10.3390/IOCC_2022-12140
Chicago/Turabian StyleRúa-Sueiro, Marcos, Paula Munín-Cruz, Juan M. Ortigueira, and José M. Vila. 2022. "X-ray Diffraction Study of Fluorine-Functionalized Thiosemicarbazones and Cyclometallated Compounds" Chemistry Proceedings 9, no. 1: 3. https://doi.org/10.3390/IOCC_2022-12140
APA StyleRúa-Sueiro, M., Munín-Cruz, P., Ortigueira, J. M., & Vila, J. M. (2022). X-ray Diffraction Study of Fluorine-Functionalized Thiosemicarbazones and Cyclometallated Compounds. Chemistry Proceedings, 9(1), 3. https://doi.org/10.3390/IOCC_2022-12140






