Fabrication and Characterization of Ethylammonium- and Rubidium-Added Perovskite Solar Cells †
Abstract
:1. Introduction
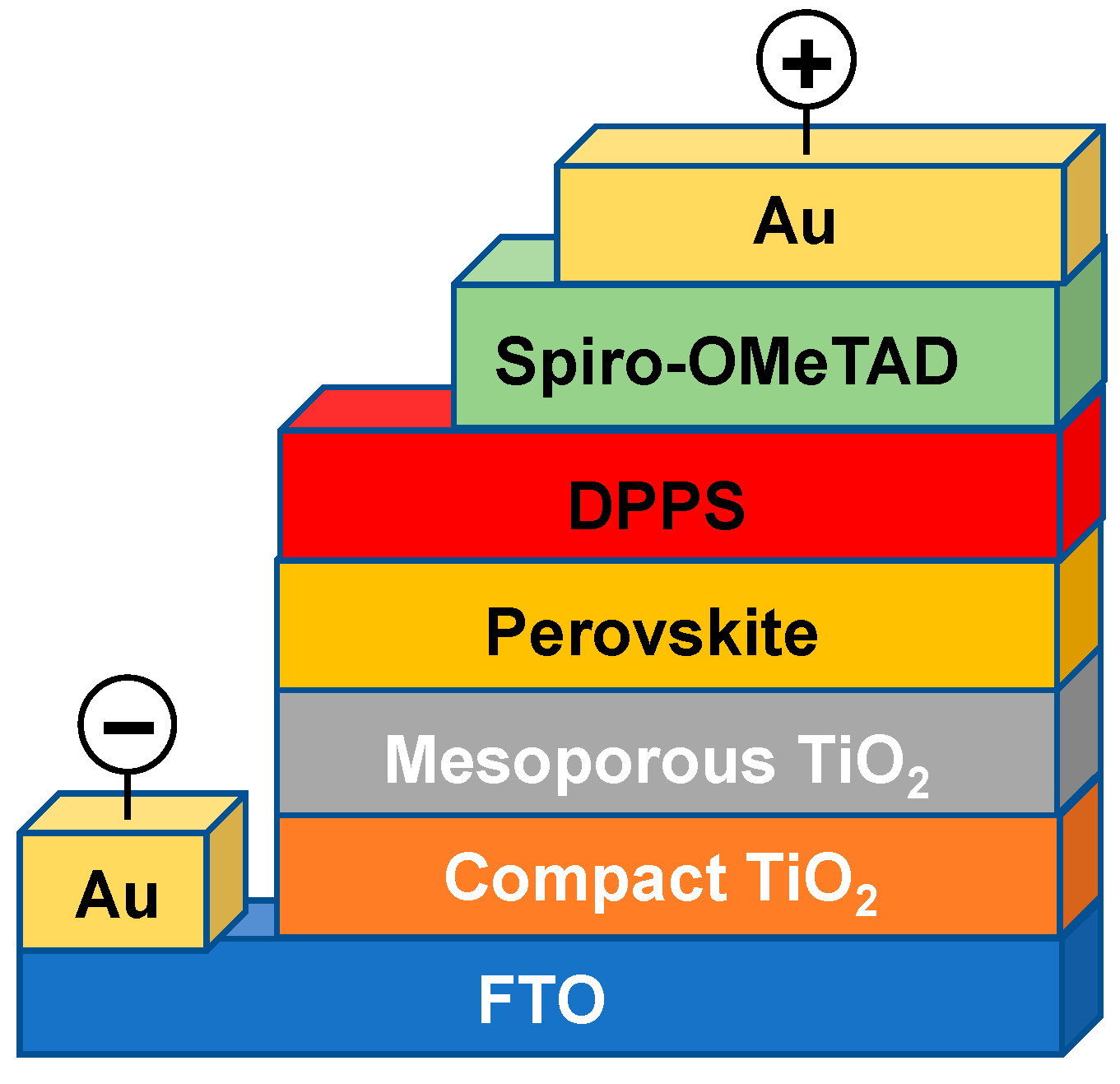
2. Experimental
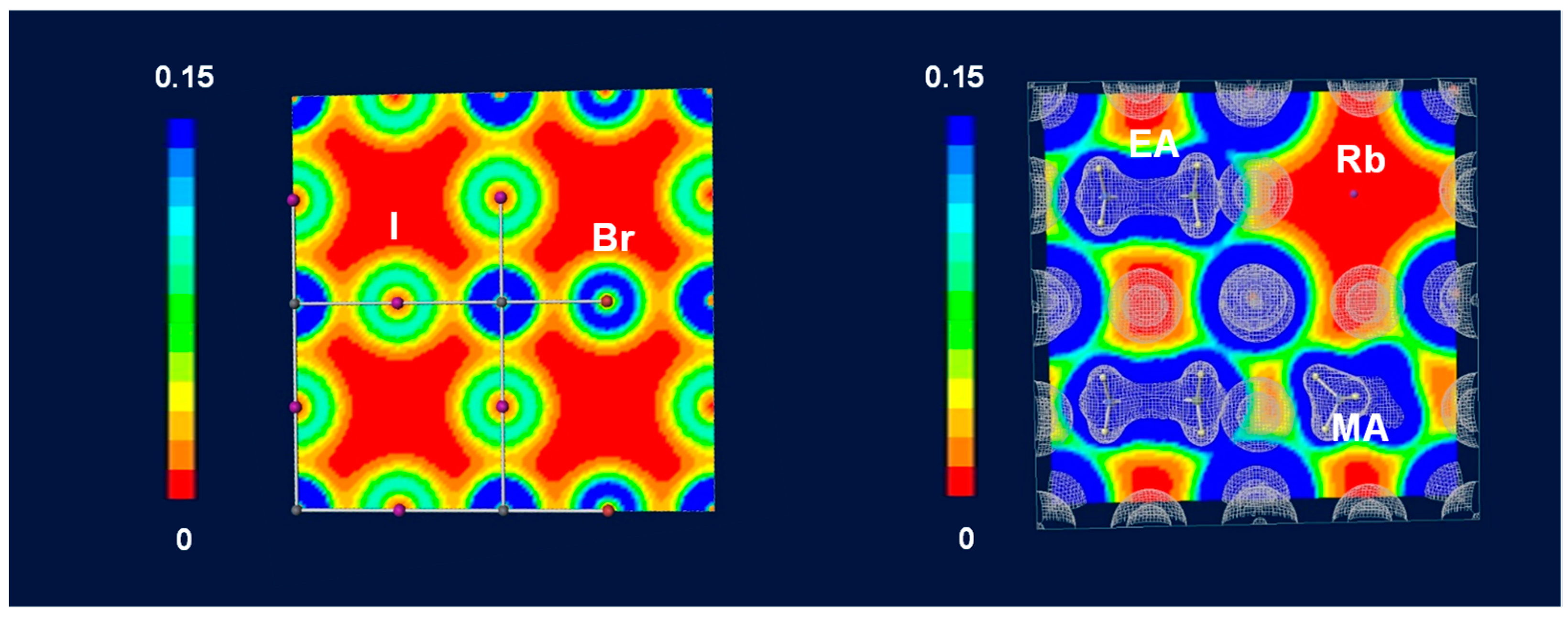
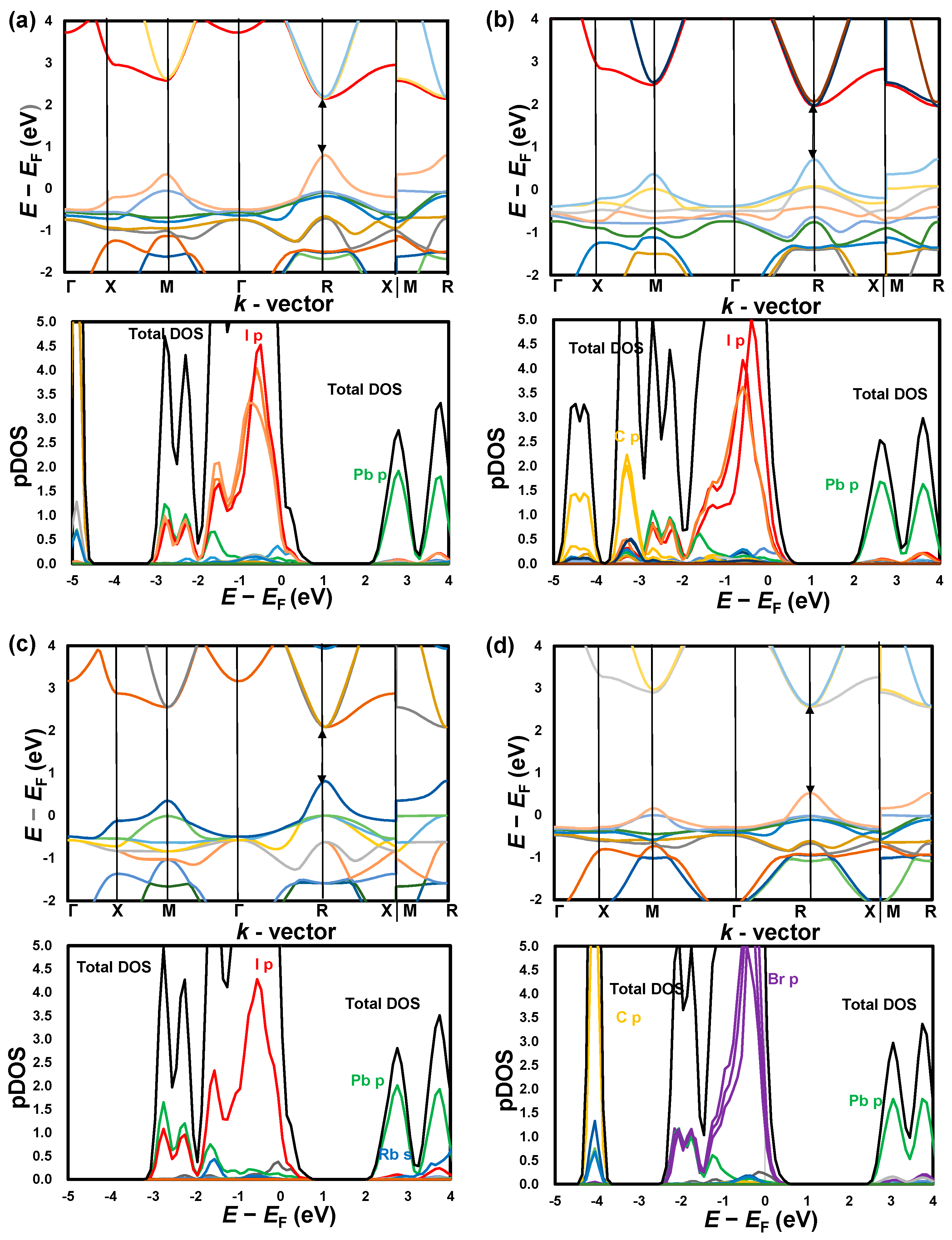
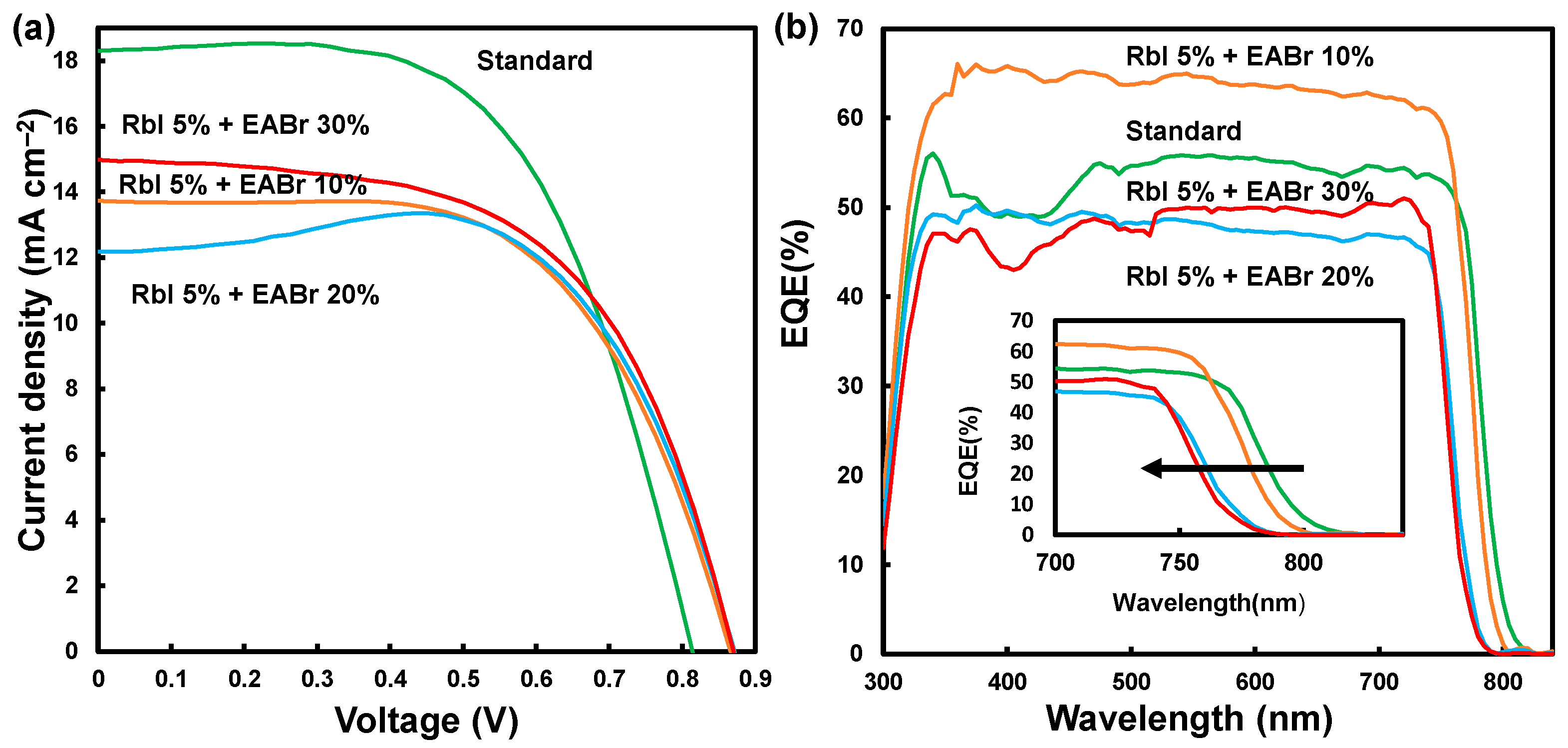
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, Y.; Zhang, T.; Li, G.; Xu, F.; Li, Y.; Yang, Y.; Zhao, Y. A mixed-cation lead iodide MA1−xEAxPbI3 absorber for perovskite solar cells. J. Energy Chem. 2018, 27, 215–218. [Google Scholar] [CrossRef] [Green Version]
- Matsui, T.; Yokoyama, T.; Negami, T.; Sekiguchi, T.; Saliba, M.; Grätzel, M.; Sagawa, H. Effect of rubidium for thermal stability of triple-cation perovskite solar cells. Chem. Lett. 2018, 12, 814–816. [Google Scholar] [CrossRef]
- Zhang, M.; Yun, J.S.; Ma, Q.; Zheng, J.; Lau, C.F.J.; Deng, X.; Kim, J.; Kim, D.; Seidel, J.; Green, M.A.; et al. High-efficiency rubidium-incorporated perovskite solar cells by gas quenching. ACS Energy Lett. 2017, 2, 438–444. [Google Scholar] [CrossRef]
- Fei, C.; Li, B.; Zhang, R.; Fu, H.; Tian, J.; Cao, G. Highly efficient and stable perovskite solar cells based on monolithically grained CH3NH3PbI3 film. Adv. Energy Mater. 2017, 7, 1602017. [Google Scholar] [CrossRef]
- Tailor, N.; Abdi-Jalebi, M.; Gupta, V.; Hu, H.; Dar, M.; Satapathi, G.L. Recent progress in morophology optimization in perovskite solar cell. J. Mater. Chem. A 2020, 8, 21356–21386. [Google Scholar] [CrossRef]
- Alharbi, E.A.; Alyamani, A.Y.; Kubicki, D.J.; Uhl, A.R.; Walder, B.J.; Alanazi, A.Q.; Luo, J.; Burgos-Caminal, A.; Albadri, A.; Albrithen, H.; et al. Atomic-level passivation mechanism of ammonium salts enabling highly efficient perovskite solar cells. Nat. Commun. 2019, 10, 3008. [Google Scholar] [CrossRef] [Green Version]
- Yamada, Y.; Yamada, T.; Kanemitsu, Y. Free carrier radiative recombination and photon recycling in lead halide perovskite solar cell materials. Bull. Chem. Soc. Jpn. 2017, 90, 1129–1140. [Google Scholar] [CrossRef]
- Liu, D.; Li, Q. Ethylammonium as an alternative cation for efficient perovskite solar cells from first-principles calculations. RSC Adv. 2019, 9, 7356–7361. [Google Scholar] [CrossRef] [Green Version]
- Solanki, A.; Yadav, P.; Turren-Cruz, S.H.; Lim, S.S.; Salibac, M.; Sum, T.C. Cation influence on carrier dynamics in perovskite solar cells. Nano Energy 2019, 58, 604–611. [Google Scholar] [CrossRef]
- Tu, Y.; Wu, J.; Lan, Z.; He, X.; Dong, J.; Jia, J.; Guo, P.; Lin, J.; Huang, M.; Huang, Y. Modulated CH3NH3PbI3−xBrx film for efficient perovskite solar cells exceeding 18%. Sci. Rep. 2017, 7, 444603. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.H.; Guan, C.K.; Lee, P.H.; Huang, H.C.; Li, C.F.; Huang, Y.C.; Su, W. Toward All Slot-Die Fabricated high efficiency large area perovskite solar cell using rapid near infrared heating in ambient air. Adv. Energy Mater. 2020, 10, 2001567. [Google Scholar] [CrossRef]
- Lee, H.; Kumar, N.; Tyagi, B.; He, S.; Sahani, R.; Kang, J.W. Bulky organic cations engineered lead-halide perovskites: A review on dimensionality and optoelectronic applications. Mater. Today Energy 2021, 21, 100759. [Google Scholar] [CrossRef]
- Wu, T.; Qin, Z.; Wang, Y.; Wu, Y.; Chen, W.; Zhang, S.; Cai, M.; Dai, S.; Zhang, J.; Liu, J.; et al. The main progress of perovskite solar cells in 2020–2021. Nano-Micro Lett. 2021, 13, 152. [Google Scholar] [CrossRef]
- Pitriana, P.; Wungu, T.; Hidayat, R.; Herman, H. Ab-initio calculation of APbI3 (A = Li, Na, K, Rb and Cs) perovskite crystal and their lattice constants optimization using density functional theory. J. Phys. Conf. Ser. 2019, 1170, 012023. [Google Scholar] [CrossRef]
- Ueoka, N.; Oku, T.; Suzuki, A. Effects of doping with Na, K, Rb, and formamidinium cations on (CH3NH3)0.99Rb0.01Pb0.99Cu0.01I3−x(Cl, Br)x perovskite photovoltaic cells. AIP Adv. 2020, 10, 125023. [Google Scholar] [CrossRef]
- Ke, W.; Kanatzidis, M.G. Prospects for low-toxicity lead-free perovskite solar cells. Nat. Commun. 2019, 10, 965–969. [Google Scholar] [CrossRef]
- Oku, T. Crystal structures of perovskite halide compounds used for solar cells. Rev. Adv. Mater. Sci. 2020, 59, 264–305. [Google Scholar] [CrossRef]
- Ueoka, N.; Oku, T.; Suzuki, A. Additive effects of alkali metals on Cu-modified CH3NH3PbI3-δClδ photovoltaic devices. RSC Adv. 2019, 9, 24231–24240. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, A.; Oe, M.; Oku, T. Fabrication and characterization of Ni-, Co-, and Rb-incorporated CH3NH3PbI3 perovskite solar cells. J. Electron. Mater. 2021, 50, 1980–1995. [Google Scholar] [CrossRef]
- Machiba, H.; Oku, T.; Kishimoto, T.; Ueoka, N.; Suzuki, A. Fabrication and evaluation of K-doped MA0.8FA0.1K0.1PbI3(Cl) perovskite solar cells. Chem. Phys. Lett. 2019, 730, 117–123. [Google Scholar] [CrossRef]
- Kandori, S.; Oku, T.; Nishi, K.; Kishimoto, T.; Ueoka, N.; Suzuki, A. Fabrication and characterization of potassium- and formamidinium-added perovskite solar cells. J. Ceram. Soc. Jpn. 2020, 128, 805. [Google Scholar] [CrossRef]
- Ueoka, N.; Oku, T. Effects of co-addition of sodium chloride and copper (II) bromide to mixed-cation mixed-halide perovskite photovoltaic devices. ACS Appl. Energy Mater. 2020, 3, 7272–7283. [Google Scholar] [CrossRef]
- Suzuki, A.; Kato, M.; Ueoka, N.; Oku, T. Additive effect of formamidinium chloride in methylammonium lead halide compound-based perovskite solar cells. J. Electron. Mater. 2019, 48, 3900–3907. [Google Scholar] [CrossRef]
- Nishi, K.; Oku, T.; Kishimoto, T.; Ueoka, N.; Suzuki, A. Photovoltaic characteristics of CH3NH3PbI3 perovskite solar cells added with ethylammonium bromide and formamidinium iodide. Coatings 2020, 10, 410. [Google Scholar] [CrossRef] [Green Version]
- Chu, Z.; Zhao, Y.; Ma, F.; Zhang, C.X.; Deng, H.; Gao, F.; Ye, Q.; Meng, J.; Yin, Z.; Zhang, X.; et al. Large cation ethylammonium incorporated perovskite for efficient and spectra stable blue light-emitting diodes. Nat. Commun. 2020, 11, 4146. [Google Scholar] [CrossRef] [PubMed]
- Mateen, M.; Arain, Z.; Liu, X.; Iqbal, A.; Ren, Y.; Zhang, X.; Liu, C.; Chen, Q.; Ma, S.; Ding, Y.; et al. Boosting optoelectronic performance of MAPbI3 perovskite solar cells via ethylammonium chloride additive engineering. Sci. China Mater. 2020, 63, 2477–2486. [Google Scholar] [CrossRef]
- Kishimoto, T.; Suzuki, A.; Ueoka, N.; Oku, T. Effects of guanidinium addition to CH3NH3PbI3-xClx perovskite photovoltaic devices. J. Ceram. Soc. Jpn. 2019, 127, 491–497. [Google Scholar] [CrossRef] [Green Version]
- Kishimoto, T.; Oku, T.; Suzuki, A.; Ueoka, N. Additive effects of guanidinium iodide on CH3NH3PbI3 perovskite solar cells. Phys. Status Solidi A 2021, 218, 2100396. [Google Scholar] [CrossRef]
- Ono, I.; Oku, T.; Suzuki, A.; Asakawa, Y.; Terada, S.; Okita, M.; Fukunishi, S.; Tachikawa, T. Fabrication and characterization of CH3NH3PbI3 solar cells with added guanidinium and inserted with decaphenylpentasilane. Jpn. J. Appl. Phys. 2022, 61, SB1024. [Google Scholar] [CrossRef]
- Oku, T.; Ohishi, Y. Effects of annealing on CH3NH3PbI3(Cl) perovskite photovoltaic devices. J. Ceram. Soc. Jpn. 2018, 126, 56–60. [Google Scholar] [CrossRef] [Green Version]
- Liao, K.; Li, C.; Xie, L.; Yuan, Y.; Wang, S.; Cao, Z.; Ding, L.; Hao, F. Hot-casting large-grain perovskite film for efficient solar cells: Film formation and device performance. Nano-Micro Lett. 2021, 12, 156. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, W.; Ma, B.; Shen, W.; Liu, L.; Cao, K.; Chen, S.; Huang, W. Lead-free perovskite materials for solar cells. Nano-Micro Lett. 2021, 13, 62. [Google Scholar] [CrossRef] [PubMed]
- Shao, S.; Dong, J.; Duim, H.; Brink, G.H.; Blake, G.R.; Portale, G.; Loi, M.A. Enhancing the crystallinity and perfecting the orientation of formamidinium tin iodide for highly efficient Sn-based perovskite solar cells. Nano Energy 2019, 60, 810–816. [Google Scholar] [CrossRef]
- Zhang, Y.; Kim, S.G.; Lee, D.; Shin, H.; Park, N.G. Bifacial stamping for high efficiency perovskite solar cells. Energy Environ. Sci. 2019, 12, 308–321. [Google Scholar] [CrossRef]
- Lee, J.W.; Kim, S.G.; Yang, J.M. Verification and mitigation of ion migration in perovskite solar cells. APL Mater. 2019, 7, 041111. [Google Scholar] [CrossRef] [Green Version]
- Oku, T.; Zushi, M.; Imanishi, Y.; Suzuki, A.; Suzuki, K. Microstructures and photovoltaic properties of perovskite-type CH3NH3PbI3 compounds. Appl. Phys. Express 2014, 7, 121601. [Google Scholar] [CrossRef]
- Oku, T.; Ohishi, Y.; Ueoka, N. Highly (100)-oriented CH3NH3PbI3(Cl) perovskite solar cells prepared with NH4Cl using an air blow method. RSC Adv. 2018, 8, 10389–10395. [Google Scholar] [CrossRef] [Green Version]
- Taguchi, M.; Suzuki, A.; Oku, T.; Ueoka, N.; Minami, S.; Okita, M. Effects of annealing temperature on decaphenylcyclopentasilane-inserted CH3NH3PbI3 perovskite solar cells. Chem. Phys. Lett. 2019, 737, 136822. [Google Scholar] [CrossRef]
- Oku, T.; Kandori, S.; Taguchi, M.; Suzuki, A.; Okita, M.; Minami, S.; Fukunishi, S.; Tachikawa, T. Polysilane-inserted methylammonium lead iodide perovskite solar cells doped with formamidinium and potassium. Energies 2020, 13, 4776. [Google Scholar] [CrossRef]
- Suzuki, A.; Taguchi, M.; Oku, T.; Okita, M.; Minami, S.; Fukunishi, S.; Tachikawa, T. Additive effects of methyl ammonium bromide or formamidinium bromide in methylammonium lead iodide perovskite solar cells using decaphenylcyclopentasilane. J. Mater. Sci. Mater. Electron. 2021, 32, 26449–26464. [Google Scholar] [CrossRef]
- Oku, T.; Taguchi, M.; Suzuki, A.; Kitagawa, K.; Asakawa, Y.; Yoshida, S.; Okita, M.; Minami, S.; Fukunishi, S.; Tachikawa, T. Effects of polysilane addition to chlorobenzene and high temperature annealing on CH3NH3PbI3 perovskite photovoltaic devices. Coatings 2021, 11, 665. [Google Scholar] [CrossRef]
- Han, T.H.; Lee, J.W.; Choi, C.; Tan, S.; Lee, C.; Zhao, Y.; Dai, Z.; Marco, N.D.; Lee, S.J.; Bae, S.H.; et al. Perovskite-polymer composite cross-linker approach for highly-stable and efficient perovskite solar cells. Nat. Commun. 2019, 10, 520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, A.; Oku, T. First-principles calculation study of electronic structures of alkali metals (Li, K, Na and Rb)-incorporated formamidinium lead halide perovskite compounds. Appl. Surf. Sci. 2019, 483, 912–921. [Google Scholar] [CrossRef]
- Suzuki, A.; Miyamoto, Y.; Oku, T. Electronic structures, spectroscopic properties, and thermodynamic characterization of sodium- or potassium-incorporated CH3NH3PbI3 by first-principles calculation. J. Mater. Sci. 2020, 55, 9728–9738. [Google Scholar] [CrossRef]
- Suzuki, A.; Oku, T. Effects of mixed-valence states of Eu-doped FAPbI3 perovskite crystals studied by first-principles calculation. Mater. Adv. 2021, 2, 2609–2616. [Google Scholar] [CrossRef]
- Suzuki, A.; Kitagawa, K.; Oku, T.; Okita, M.; Fukunishi, S.; Tachikawa, T. Additive effects of copper and alkali metal halides into methylammonium lead iodide perovskite solar cells. Electron. Mater. Lett. 2022, 18, 176–186. [Google Scholar] [CrossRef]
- Enomoto, A.; Suzuki, A.; Oku, T.; Okita, M.; Fukunishi, S.; Tachikawa, T.; Hasegawa, T. Effects of Cu, K and guanidinium addition to CH3NH3PbI3 perovskite solar cells. J. Electron. Mater. 2022. [Google Scholar] [CrossRef]
- Okumura, R.; Oku, T.; Suzuki, A.; Okita, M.; Fukunishi, S.; Tachikawa, T.; Hasegawa, T. Effects of adding alkali metals and organic cations to Cu-based perovskite solar cells. Appl. Sci. 2022, 12, 1710. [Google Scholar] [CrossRef]
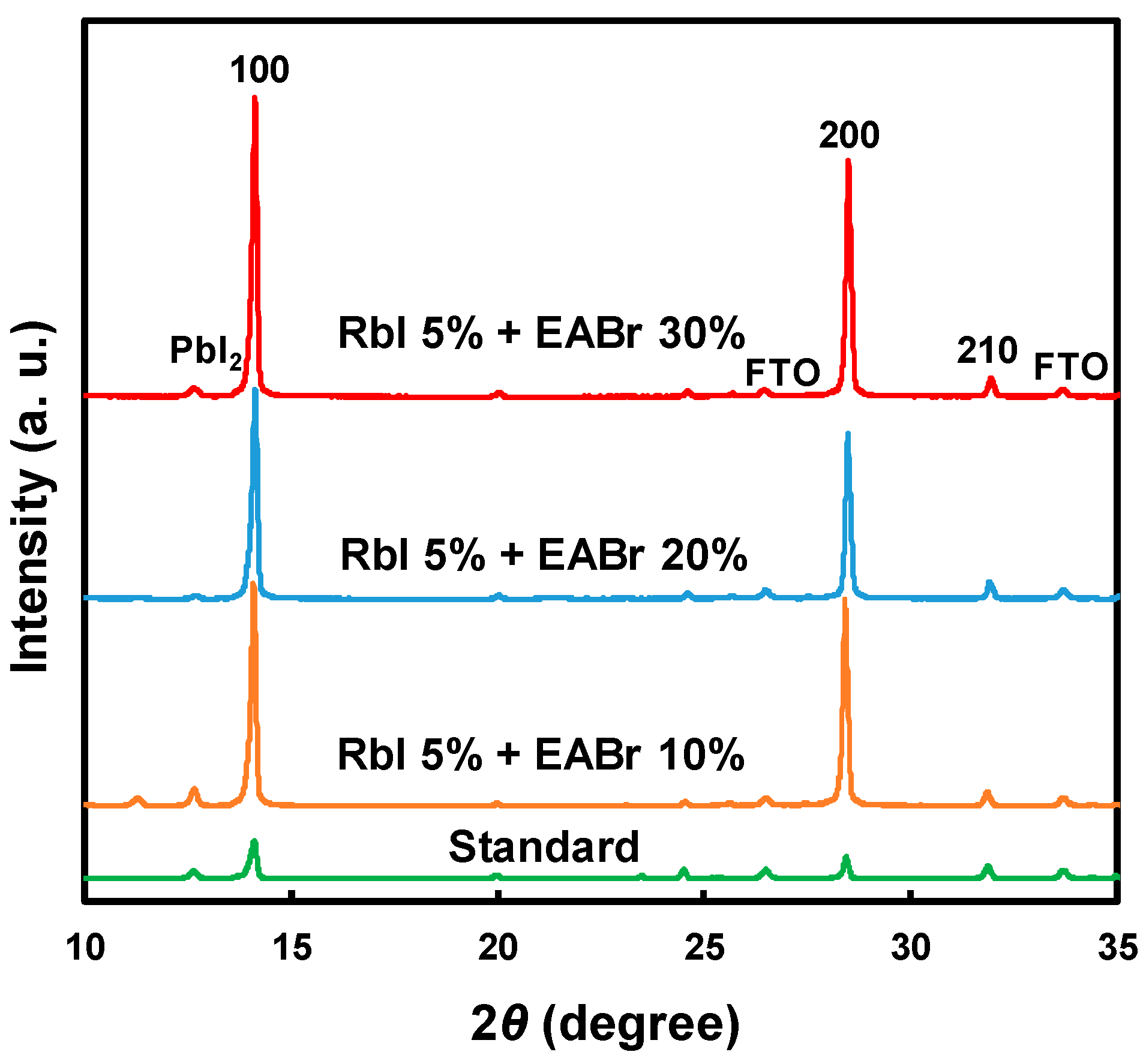
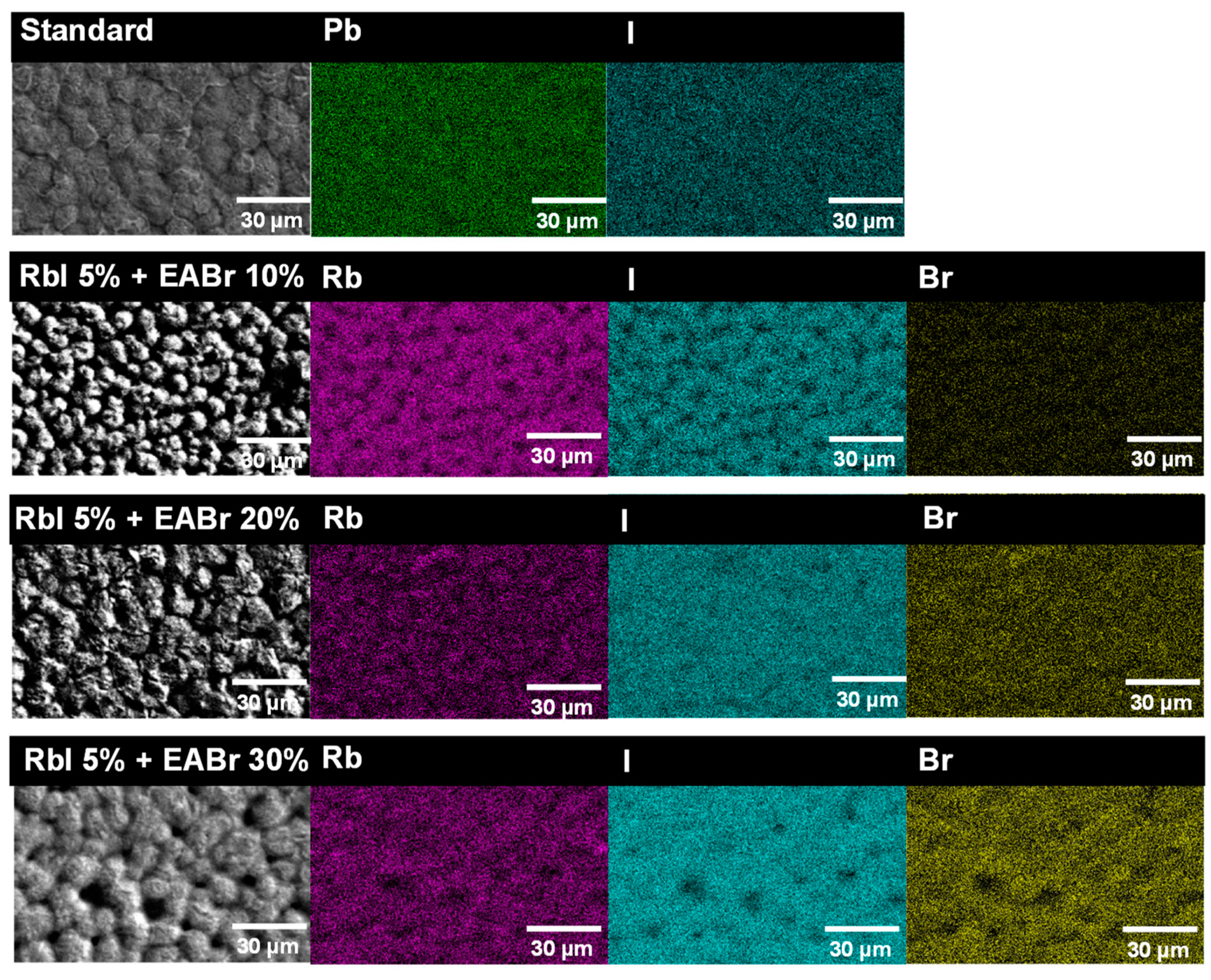
| Device | Effective Mass Ratio | Energy Gap Eg (eV) | Total Energy (eV cell−1) | |
|---|---|---|---|---|
| me */m0 | mh */m0 | |||
| MAPbI3 | 0.055 | 0.031 | 1.391 | −3483 |
| EAPbI3 | 0.049 | 0.024 | 1.241 | −3682 |
| RbPbI3 | 0.050 | 0.028 | 1.268 | −2999 |
| MAPbBr3 | 0.039 | 0.028 | 2.032 | −3657 |
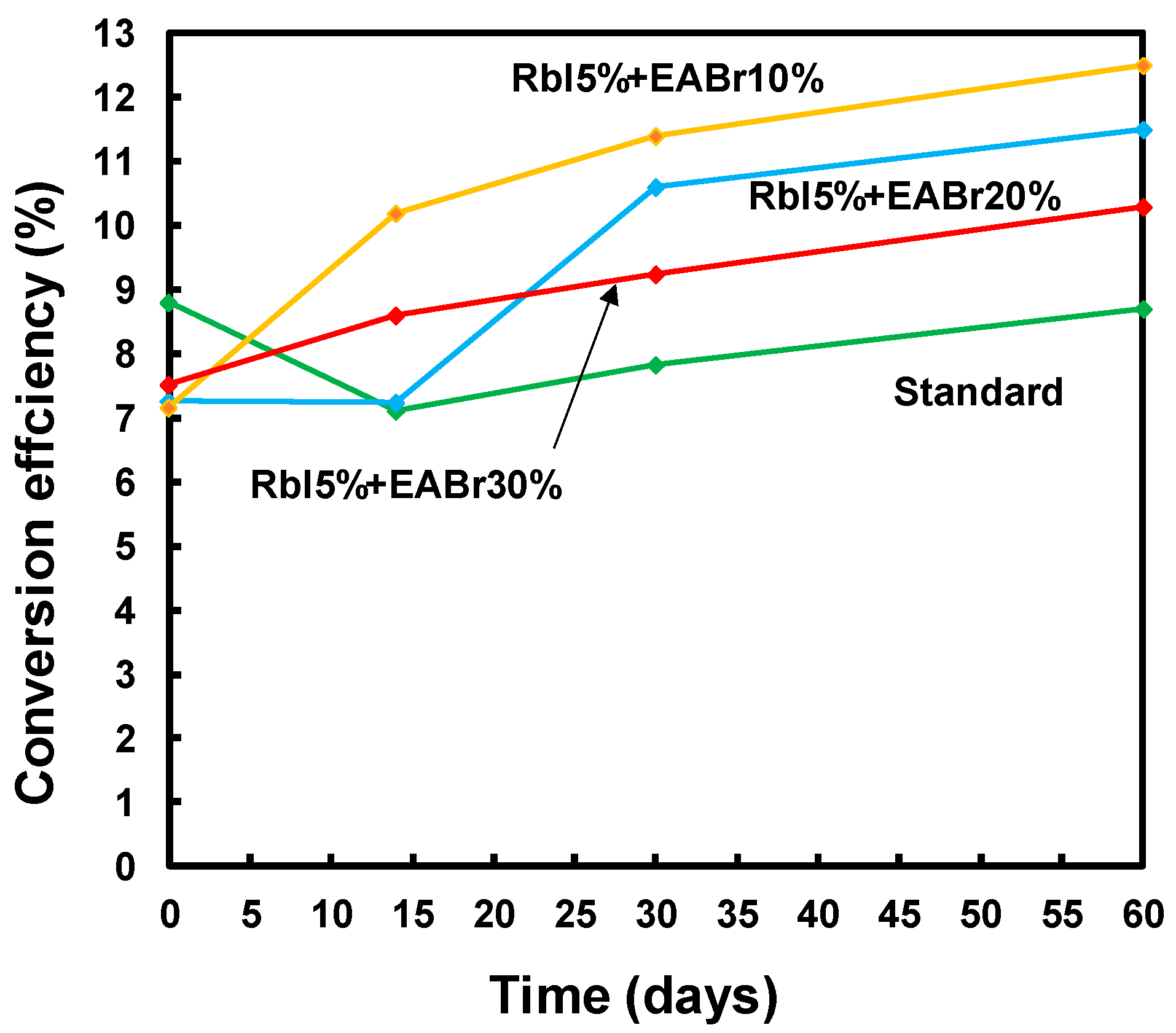
| Device | JSC (mA cm−2) | VOC (V) | FF | η (%) | ηave (%) | Rs (Ω cm2) | Rsh (Ω cm2) | Eg (eV) |
|---|---|---|---|---|---|---|---|---|
| Standard | 18.3 | 0.814 | 0.590 | 8.80 | 7.56 | 9.92 | 1427 | 1.55 |
| RbI 5% + EABr 10% | 13.7 | 0.866 | 0.602 | 7.16 | 5.92 | 13.1 | 1838 | 1.57 |
| RbI 5% + EABr 20% | 12.2 | 0.871 | 0.683 | 7.25 | 5.62 | 12.8 | 1698 | 1.60 |
| RbI 5% + EABr 30% | 15.0 | 0.869 | 0.576 | 7.51 | 6.44 | 10.8 | 1079 | 1.61 |
| After 60 days | - | - | - | - | - | - | - | - |
| Standard | 17.3 | 0.880 | 0.576 | 8.77 | 8.53 | 6.08 | 907 | 1.56 |
| RbI 5% + EABr 10% | 21.6 | 0.901 | 0.646 | 12.6 | 10.9 | 5.67 | 677 | 1.59 |
| RbI 5% + EABr 20% | 19.4 | 0.944 | 0.626 | 11.5 | 11.3 | 9.12 | 1030 | 1.61 |
| RbI 5% + EABr 30% | 17.7 | 0.933 | 0.622 | 10.3 | 9.89 | 7.99 | 1290 | 1.62 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takada, K.; Oku, T.; Suzuki, A.; Okita, M.; Fukunishi, S.; Tachikawa, T.; Hasegawa, T. Fabrication and Characterization of Ethylammonium- and Rubidium-Added Perovskite Solar Cells. Chem. Proc. 2022, 9, 14. https://doi.org/10.3390/IOCC_2022-12153
Takada K, Oku T, Suzuki A, Okita M, Fukunishi S, Tachikawa T, Hasegawa T. Fabrication and Characterization of Ethylammonium- and Rubidium-Added Perovskite Solar Cells. Chemistry Proceedings. 2022; 9(1):14. https://doi.org/10.3390/IOCC_2022-12153
Chicago/Turabian StyleTakada, Keinoshin, Takeo Oku, Atsushi Suzuki, Masanobu Okita, Sakiko Fukunishi, Tomoharu Tachikawa, and Tomoya Hasegawa. 2022. "Fabrication and Characterization of Ethylammonium- and Rubidium-Added Perovskite Solar Cells" Chemistry Proceedings 9, no. 1: 14. https://doi.org/10.3390/IOCC_2022-12153
APA StyleTakada, K., Oku, T., Suzuki, A., Okita, M., Fukunishi, S., Tachikawa, T., & Hasegawa, T. (2022). Fabrication and Characterization of Ethylammonium- and Rubidium-Added Perovskite Solar Cells. Chemistry Proceedings, 9(1), 14. https://doi.org/10.3390/IOCC_2022-12153






