Covalent Binding of C60 Fullerene to Quadricyclanes A Synthetic Avenue to Hexamethanofullerenes with Promising Antitumor Activity †
Abstract
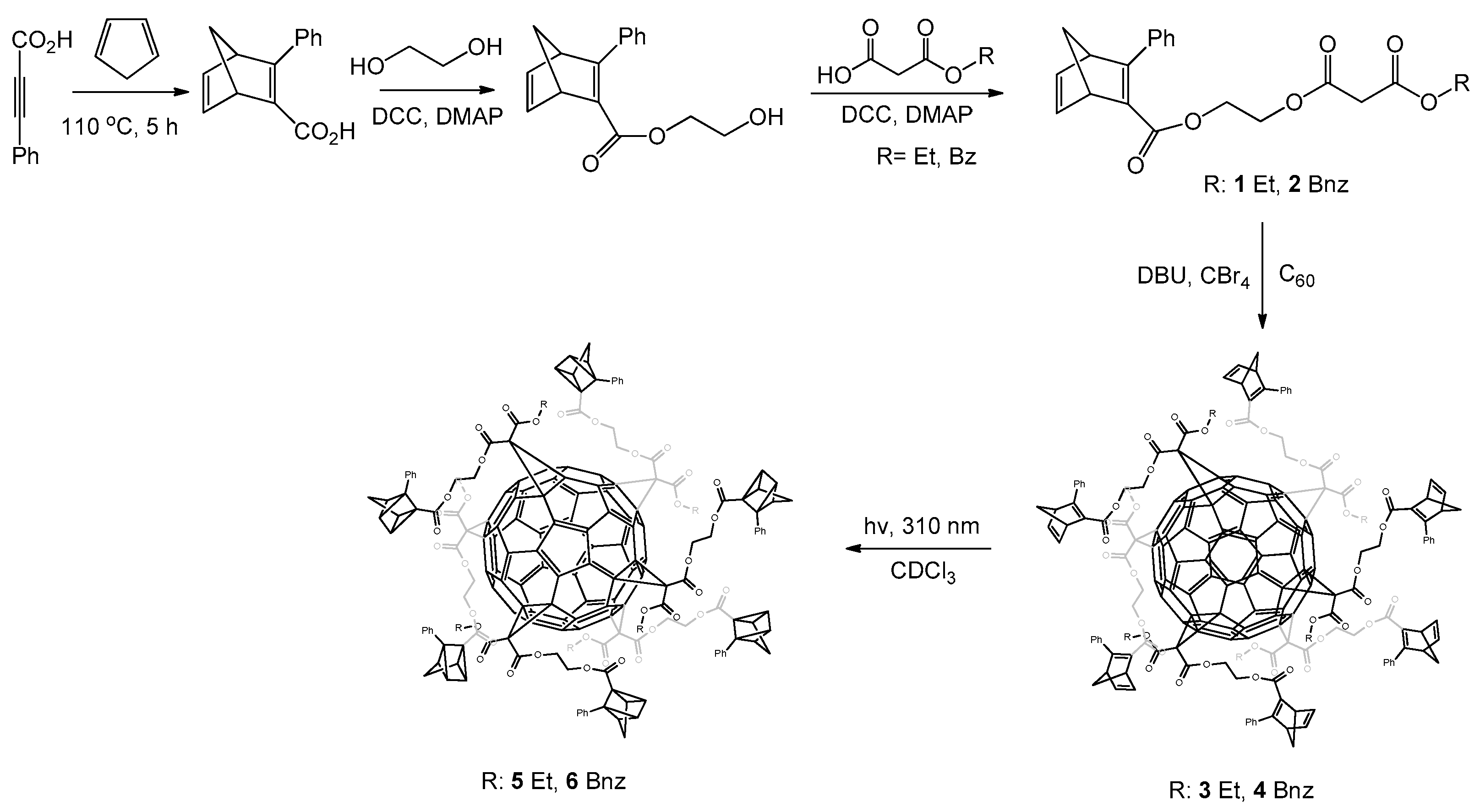
:1. Introduction
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- An, X.; Xie, Y. Enthalpy of isomerization of quadricyclane to norbornadiene. Thermochim. Acta 1993, 220, 17–25. [Google Scholar] [CrossRef]
- Wiberg, K.B.; Connon, H.A. Enthalpy of the metal catalyzed isomerizations of quadricyclane and of tricyclo[4.1.0.02,7]heptane. J. Am. Chem. Soc. 1976, 98, 5411–5412. [Google Scholar] [CrossRef]
- Dzhemilev, U.M.; Akhmetov, A.R.; Khuzin, A.A.; D’yakonov, V.A.; Dzhemileva, L.U.; Yunusbaeva, M.M.; Khalilov, L.M.; Tuktarov, A.R. A new original approach to the design of anticancer drugs based on energy-rich quadricyclanes. Russ. Chem. Bull. 2019, 5, 1036–1040. [Google Scholar] [CrossRef]
- Liu, J.H.; Cao, L.; Luo, P.G.; Yang, S.T.; Lu, F.; Wang, H.; Meziani, M.J.; Haque, S.A.; Liu, Y.; Lacher, S.; et al. Fullerene-conjugated doxorubicin in cells. ACS Appl. Mater. Interfaces 2010, 2, 1384–1389. [Google Scholar] [CrossRef] [PubMed]
- Prylutska, S.; Panchuk, R.; Gołuński, G.; Skivka, L.; Prylutskyy, Y.; Hurmach, V.; Skorohyd, N.; Borowik, A.; Woziwodzka, A.; Piosik, J.; et al. C60 fullerene enhances cisplatin anticancer activity andovercomes tumor cell drug resistance. Nano Res. 2017, 10, 652–671. [Google Scholar] [CrossRef] [Green Version]
- Dzhemilev, U.M.; Khuzin, A.A.; Akhmetov, A.R.; D’yakonov, V.A.; Dzhemileva, L.U.; Yunusbaeva, M.M.; Tuktarov, A.R. Synthesis of C60 fullerene-quadricyclane hybrid compound and its preliminary in vitro antitumor activity in combination with cisplatin. ACS Omega 2019, 4, 15929–15934. [Google Scholar] [CrossRef] [Green Version]
- Tuktarov, A.R.; Akmetov, A.R.; Khuzin, A.A.; Dzhemilev, U.M. Synthesis and properties of energy-rich methanofullerenes containing quadricyclane moieties. J. Org. Chem. 2018, 83, 4160–4166. [Google Scholar] [CrossRef]
- Dzhemilev, U.M.; Akhmetov, A.R.; D’yakonov, V.A.; Dzhemileva, L.U.; Yunusbaeva, M.M.; Tuktarov, A.R. Synthesis and antitumor activity of methanofullerenes equipped with norbornadiene and quadricyclane moieties. Mendeleev Commun. 2020, 30, 150–152. [Google Scholar] [CrossRef]
- Buffet, K.; Gillon, E.; Holler, M.; Nierengarten, J.-F.; Imberty, A.; Vincent, S.P. Fucofullerenes as tight ligands of RSL and LecB, two bacterial lectins. Org. Biomol. Chem. 2015, 13, 6482–6492. [Google Scholar] [CrossRef]
- Luczkowiak, J.; Munoz, A.; Sanchez-Navarro, M.; Ribeiro-Viana, R.; Ginieis, A.; Illescas, B.M.; Martin, N.; Delgado, R.; Rojo, J. Glycofullerenes inhibit viral infection. Biomacromolecules 2013, 14, 431–437. [Google Scholar]
- Kraevaya, O.A.; Peregudov, A.S.; Godovikov, I.A.; Shchurik, E.V.; Martynenko, V.M.; Shestakov, A.F.; Balzarini, J.; Schols, D.; Troshin, P.A. Direct arylation of C60Cl6 and C70Cl8 with carboxylic acids: A synthetic avenue to water-soluble fullerene derivatives with promising antiviral activity. Chem. Commun. 2020, 56, 1179–1182. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.-J.; Chetyrkina, M.; Wong, C.-W.; Kraevaya, O.A.; Zhilenkov, A.V.; Voronov, I.I.; Wang, P.-H.; Troshin, P.A.; Hsu, S.-H. Identification of potential descriptors of water-soluble fullerene derivatives responsible for antitumor effects on lung cancer cells via QSAR analysis. Comput. Struct. Biotechnol. J. 2021, 19, 812–825. [Google Scholar] [CrossRef] [PubMed]
- Troshina, O.A.; Troshin, P.A.; Peregudov, A.S.; Kozlovskiy, V.I.; Balzarini, J.; Lyubovskaya, R.N. Chlorofullerene C60Cl6: A precursor for straightforward preparation of highly water-soluble polycarboxylic fullerene derivatives active against HIV. Org. Biomol. Chem. 2007, 5, 2783–2791. [Google Scholar] [CrossRef]
- Leng, F.; Gerber, I.C.; Lecante, P.; Bentaleb, A.; Muñoz, A.; Illescas, B.M.; Martín, N.; Melinte, G.; Ersen, O.; Martinez, H.; et al. Hexakis [60]Fullerene Adduct-Mediated Covalent Assembly of Ruthenium Nanoparticles and Their Catalytic Properties. Chem. A Eur. J. 2017, 23, 13379–13386. [Google Scholar] [CrossRef] [PubMed]
- Iehl, J.; Freitas, R.P.; Nicot, B.D.; Nierengarten, J.-F. Click chemistry for the efficient preparation of functionalized [60]fullerene hexakis-adducts. Chem. Commun. 2008, 21, 2450–2452. [Google Scholar] [CrossRef] [PubMed]
- Soriano, J.R.; Reina, J.J.; Illescas, B.M.; Rojo, J.; Martín, N. Maleimide and Cyclooctyne-Based Hexakis-Adducts of Fullerene: Multivalent Scaffolds for Copper-Free Click Chemistry on Fullerenes. J. Org. Chem. 2018, 83, 1727–1736. [Google Scholar] [CrossRef]
- Lorenz, P.; Hirsch, A. Photoswitchable norbornadiene/quadricyclane interconversion-mediated by covalently linked C60. Chem. Eur. J. 2020, 26, 5220–5230. [Google Scholar] [CrossRef]
- Hirsch, A.; Vostrowsky, O. C60 Hexakisadducts with an Octahedral Addition Pattern—A New Structure Motif in Organic Chemistry. Eur. J. Org. Chem. 2001, 2001, 829–848. [Google Scholar] [CrossRef]
- Dubonosov, A.D.; Bren, V.A.; Chernoivanov, V.A. Norbornadiene—Quadricyclane as an abiotic system for the storage of solar energy. Russ. Chem. Rev. 2002, 71, 917–927. [Google Scholar] [CrossRef]
- Petersen, A.U.; Jevric, M.; Poulsen, K.M. Triazole functionalized Norbornadiene-Quadricyclane Photoswitches for Solar Energy Storage. Eur. J. Org. Chem. 2018, 32, 4465–4474. [Google Scholar] [CrossRef]
- Andreev, S.; Purgina, D.; Bashkatova, E.; Garshev, A.; Maerle, A.; Andreev, I.; Osipova, N.; Shershakova, N.; Khaitov, M. Study of Fullerene Aqueous Dispersion Prepared by Novel Dialysis Method: Simple Way to Fullerene Aqueous Solution. Fuller. Nanotub. Carbon Nanostruct. 2015, 23, 792–800. [Google Scholar] [CrossRef]
- Kyzyma, O.; Bashmakova, N.; Gorshkova, Y.; Ivankov, O.; Mikheev, I.; Kuzmenko, M.; Kutovyy, S.; Nikolaienko, T. Interaction between the plant alkaloid berberine and fullerene C70: Experimental and quantum-chemical study. J. Mol. Liq. 2019, 278, 452–459. [Google Scholar] [CrossRef]
- Mikheev, I.V.; Pirogova, M.O.; Usoltseva, L.O.; Uzhel, A.S.; Bolotnik, T.A.; Kareev, I.E.; Bubnov, V.P.; Lukonina, N.S.; Volkov, D.S.; Goryunkov, A.A.; et al. Green and rapid preparation of long-term stable aqueous dispersions of fullerenes and endohedral fullerenes: The pros and cons of an ultrasonic probe. Ultrason. Sonochem. 2021, 73, 105533–105543. [Google Scholar] [CrossRef] [PubMed]
- Mchedlov-Petrossyan, N.O. Fullerenes in Liquid Media: An Unsettling Intrusion into the Solution Chemistry. Chem. Rev. 2013, 113, 5149–5193. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akhmetov, A.; Sadretdinova, Z.; Dzhemileva, L.U.; Tuktarov, A.; Dzhemilev, U. Covalent Binding of C60 Fullerene to Quadricyclanes A Synthetic Avenue to Hexamethanofullerenes with Promising Antitumor Activity. Chem. Proc. 2022, 8, 95. https://doi.org/10.3390/ecsoc-25-11649
Akhmetov A, Sadretdinova Z, Dzhemileva LU, Tuktarov A, Dzhemilev U. Covalent Binding of C60 Fullerene to Quadricyclanes A Synthetic Avenue to Hexamethanofullerenes with Promising Antitumor Activity. Chemistry Proceedings. 2022; 8(1):95. https://doi.org/10.3390/ecsoc-25-11649
Chicago/Turabian StyleAkhmetov, Arslan, Zarema Sadretdinova, Lilya U. Dzhemileva, Airat Tuktarov, and Usein Dzhemilev. 2022. "Covalent Binding of C60 Fullerene to Quadricyclanes A Synthetic Avenue to Hexamethanofullerenes with Promising Antitumor Activity" Chemistry Proceedings 8, no. 1: 95. https://doi.org/10.3390/ecsoc-25-11649
APA StyleAkhmetov, A., Sadretdinova, Z., Dzhemileva, L. U., Tuktarov, A., & Dzhemilev, U. (2022). Covalent Binding of C60 Fullerene to Quadricyclanes A Synthetic Avenue to Hexamethanofullerenes with Promising Antitumor Activity. Chemistry Proceedings, 8(1), 95. https://doi.org/10.3390/ecsoc-25-11649






