Synthesis of Bis-Hydrazine Using Heterogeneous Catalysis †
Abstract
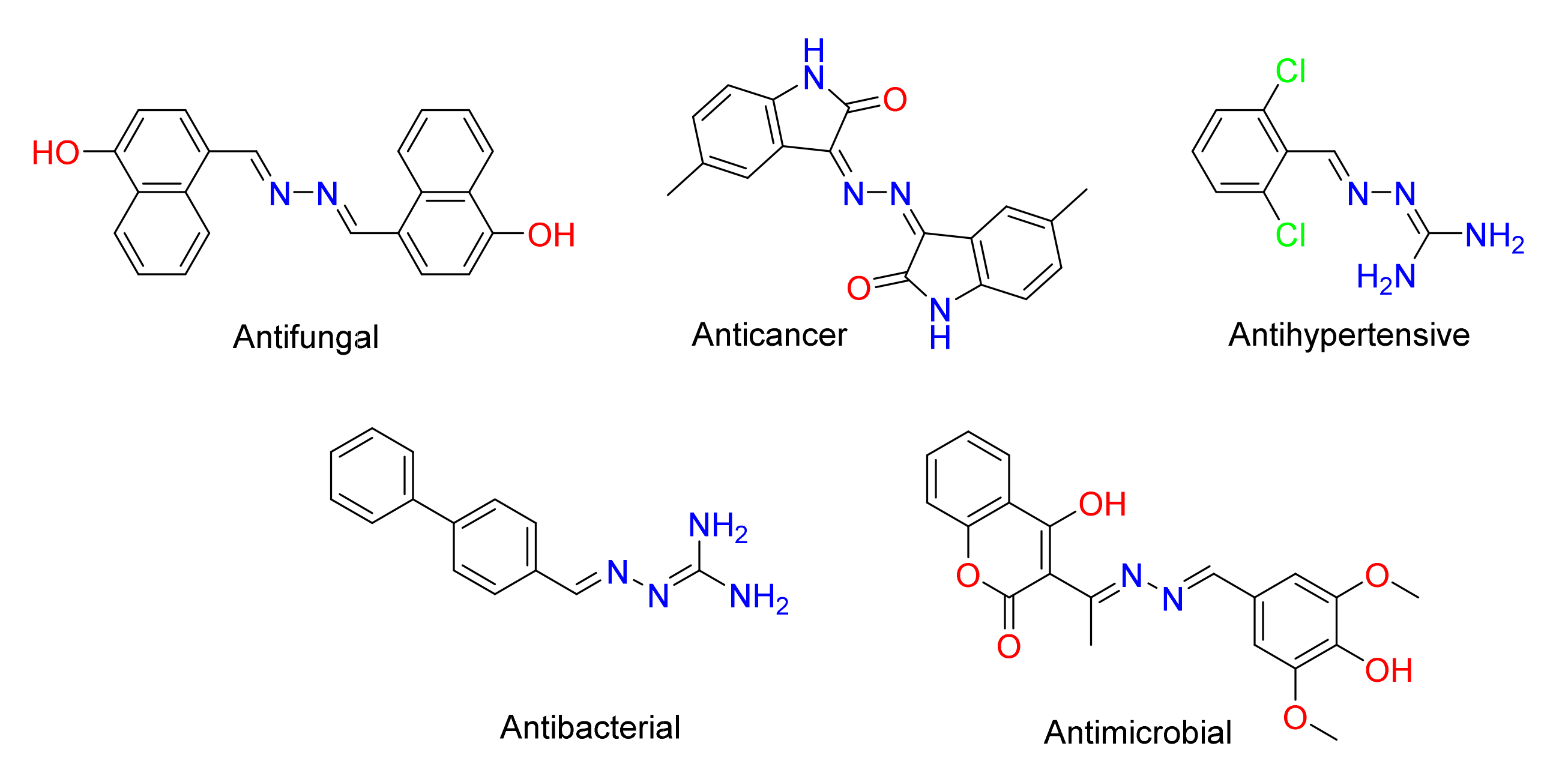
:1. Introduction
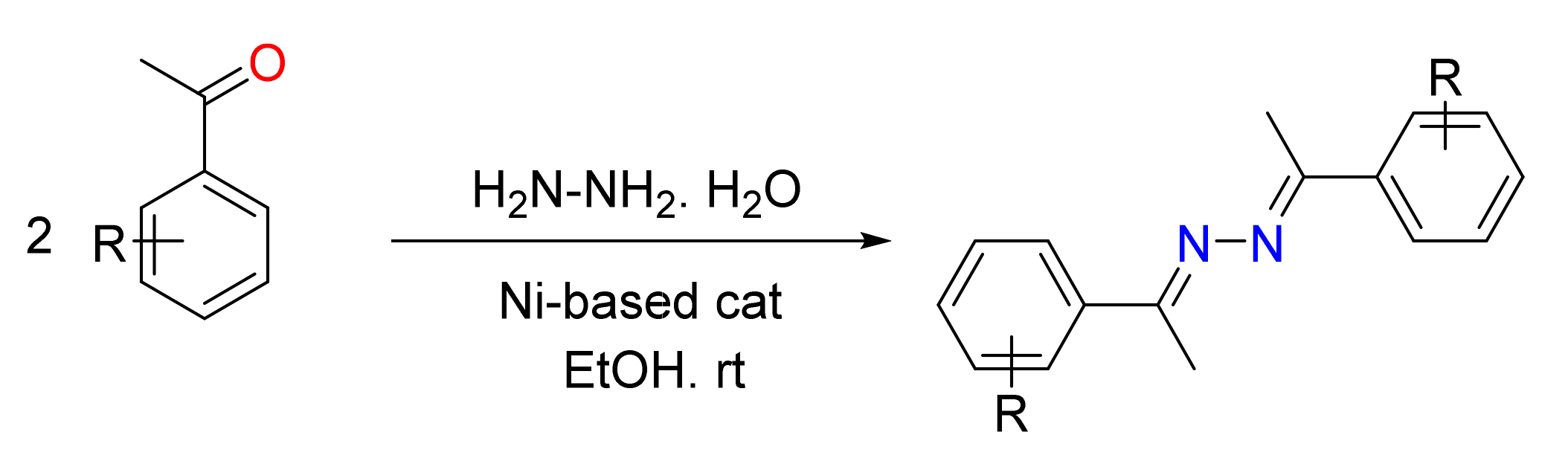
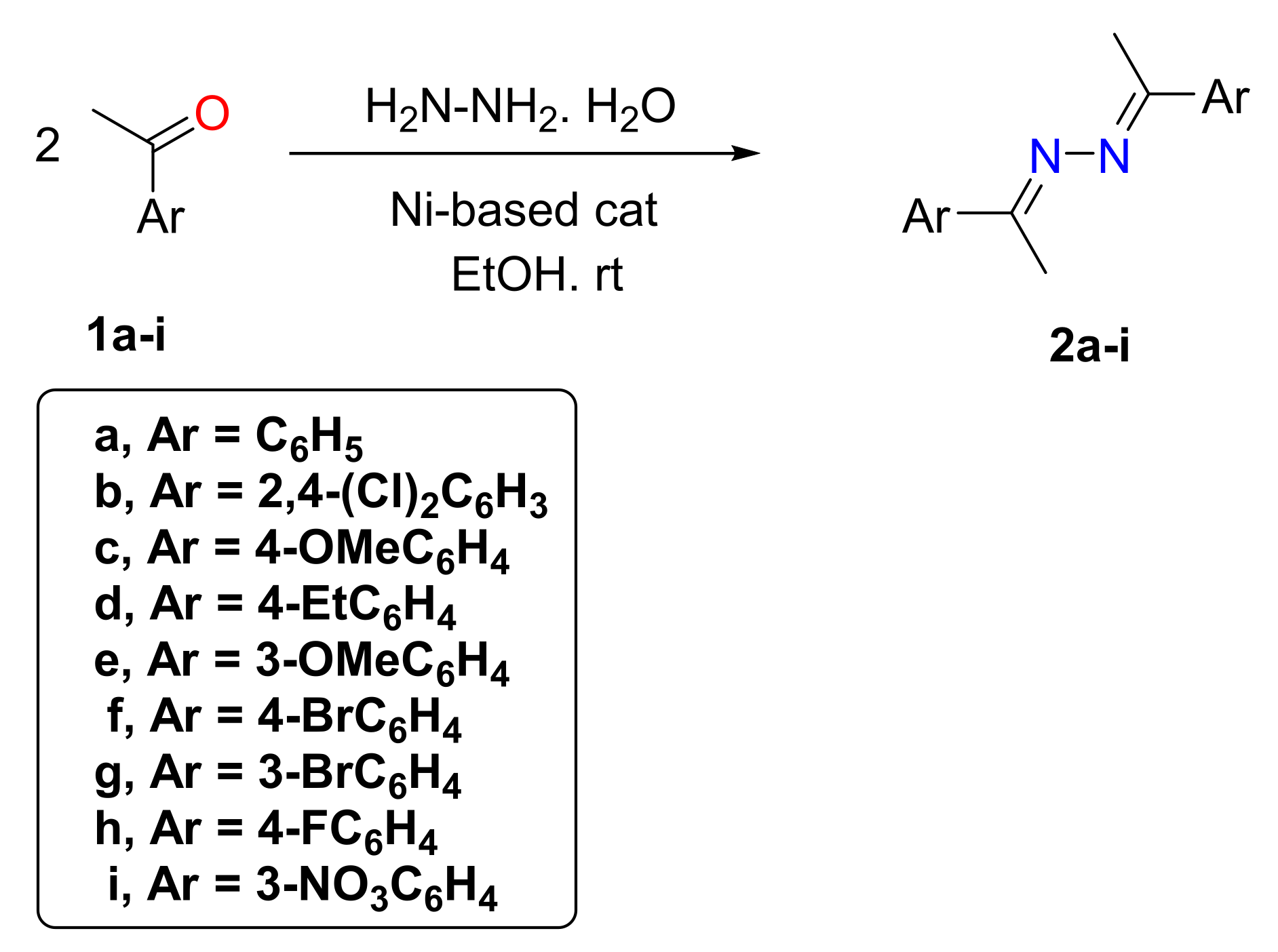
2. General Experimental Procedure
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Becker, C. From Langmuir to Ertl: The “Nobel” History of the Surface Science Approach to Heterogeneous Catalysis. In Encyclopedia of Interfacial Chemistry; Elsevier: Amsterdam, The Netherlands, 2018; pp. 99–106. [Google Scholar]
- Dömling, A.; Wang, W.; Wang, K. Chemistry and Biology Of Multicomponent Reactions. Chem. Rev. 2012, 112, 3083–3135. [Google Scholar] [CrossRef] [Green Version]
- Poliakoff, M. Green Chemistry: Science and Politics of Change. Science 2002, 297, 807–810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sumran, G.; Aggarwal, R.; Hooda, M.; Sanz, D.; Claramunt, R.M. Unusual synthesis of azines and their oxidative degradation to carboxylic acid using iodobenzene diacetate. Synth. Commun. 2018, 48, 439–446. [Google Scholar] [CrossRef]
- Huisgen, R. Cycloadditions definition, classification, and characterization. Angew. Chem. Int. Ed. Engl. 1968, 7, 321–328. [Google Scholar] [CrossRef]
- Wagner-Jauregg, T. Reaktionen von Azinen und Iminen (Azomethinen, Schiff’schenBasen) mitDienophilen. Synthesis 1976, 1976, 349–373. [Google Scholar] [CrossRef]
- Goodall, G.W.; Hayes, W. Advances in cycloaddition polymerizations. Chem. Soc. Rev. 2006, 35, 280–312. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Yao, S.; Driess, M. Unusual [3 + 1] Cycloaddition of a Stable Silylene with a 2,3-Diazabuta-1,3-diene versus [4 + 1] Cycloaddition toward a Buta-1,3-diene. Organometallics 2010, 29, 987–990. [Google Scholar] [CrossRef]
- Vyas, V.S.; Haase, F.; Stegbauer, L.; Savasci, G.; Podjaski, F.; Ochsenfeld, C.; Lotsch, B.V. A tunableazine covalent organic framework platform for visible light-induced hydrogen generation. Nat. Commun. 2015, 6, 8508. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, A.R.; Brown, K.G.; Graham, D.; Kirkhouse, J.B.; Kittner, M.; Major, C.; McHugh, C.J.; Murdoch, P.; Smith, W.E. Chromophore containing bipyridyl ligands. Part 1: Supramolecular solid-state structure of Ag(I) complexes. New J. Chem. 2005, 29, 826–832. [Google Scholar] [CrossRef]
- Dragancea, D.; Arion, V.B.; Shova, S.; Rentschler, E.; Gerbeleu, N.V. Azine-bridged octanuclearcopper(II) complexes assembled with a one-stranded ditopicthiocarbohydrazone ligand. Angew. Chem. Int. Ed. 2005, 44, 7938–7942. [Google Scholar] [CrossRef]
- Hauer, C.R.; King, G.S.; McCool, E.L.; Euler, W.B.; Ferrara, J.D.; Youngs, W.J. Structure of 2,3-butanedione dihydrazone and IR study of higher polyazines: A new class of polymeric conductors. J. Am. Chem. Soc. 1987, 109, 5760–5765. [Google Scholar] [CrossRef]
- Chaloner-Gill, B.; Cheer, C.J.; Roberts, J.E.; Euler, W.B. Structure of glyoxaldihydrazone and synthesis, characterization, and iodine doping of unsubstituted polyazine. Macromolecules 1990, 23, 4597–4603. [Google Scholar] [CrossRef]
- Martínez, R.; Espinosa, A.; Tarraga, A.; Molina, P. New Hg2+ and Cu2+ Selective Chromo- and Fluoroionophore Based on a BichromophoricAzine. Org. Lett. 2005, 7, 5869–5872. [Google Scholar] [CrossRef]
- Suresh, M.; Mandal, A.K.; Saha, S.; Suresh, E.; Mandoli, A.; Di Liddo, R.; Parnigotto, P.P.; Das, A. Azine-Based Receptor for Recognition of Hg2+ Ion: Crystallographic Evidence and Imaging Application in Live Cells. Org. Lett. 2010, 12, 5406–5409. [Google Scholar] [CrossRef]
- Centore, R.; P-nunzi, B.; Roviello, A.; Sirigu, A.; Villano, P. Synthesis, Characterisation, and Phase Behaviour of Some Azines with Potential Optical Nonlinearities of Second Order. Mol. Cryst. Liq. Cryst. Sci. Technol. Sect. A 1996, 275, 107–120. [Google Scholar] [CrossRef]
- Custodio, J.M.F.; Ternavisk, R.R.; Ferreira, C.J.S.; Figueredo, A.S.; Aquino, G.L.B.; Napolitano, H.B.; Valverde, C.; Baseia, B. Using the Supermolecule Approach To Predict the Nonlinear Optics Potential of aNovel Asymmetric Azine. J. Phys. Chem. A 2019, 123, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Ristic, M.N.; Radulovic, N.S.; Dekic, B.R.; Dekic, V.S.; Ristic, N.R.; Stojanovic-Radic, Z. Synthesis and spectral characterization of asymmetric azines containing a coumarin moiety: The discovery ofnew antimicrobial and antioxidant agents. Chem. Biodivers. 2019, 16, e1800486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nash, D.T. Clinical trial with Guanabenz, a new antihypertensive Agent. J. Clin. Pharmacol. New Drugs 1973, 13, 416–421. [Google Scholar] [CrossRef]
- Kurteva, V.B.; Simeonov, S.P.; Stoilova-Disheva, M. Symmetrical acyclic aryl aldazines with antibacterial and antifungal activity. Pharmacol. Pharm. 2011, 2, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Cavallini, G.; Massarani, E.; Nardi, D.; Mauri, L.; Mantegazza, P. Antibacterial Agents. Some New Guanyhydrazone Derivatives. J. Med. Pharm. Chem. 1961, 4, 177–182. [Google Scholar]
- Liang, C.; Xia, J.; Lei, D.; Li, X.; Yao, Q.; Gao, J. Synthesis, in vitro and in vivo antitumor activity of symmetrical bis-Schiff base derivatives of isatin. Eur. J. Med. Chem. 2014, 74, 742–750. [Google Scholar] [PubMed]
- Chourasiya, S.S.; Kathuria, D.; Wani, A.; Bharatam, P.V. Azines: Synthesis, Structure, Electronic Structure and their Applications. Org. Biomol. Chem. 2019, 17, 8486–8521. [Google Scholar] [CrossRef]
- Bauer, J.O.; Leitus, G.; Ben-David, Y.; Milstein, D. Direct Synthesis of Symmetrical Azines from Alcohols and Hydrazine Catalyzed by a Ruthenium Pincer Complex: Effect of Hydrogen Bonding. ACS Catal. 2016, 6, 8415–8419. [Google Scholar] [PubMed]
- Qiu, D.; Mo, F.; Zhang, Y.; Wang, J. Recent Advances in Transition-Metal-Catalyzed Cross-Coupling Reactions with N -Tosylhydrazones. Adv. Organomet. Chem. 2017, 67, 151–219. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medjahed, N.; Kibou, Z.; Berrichi, A.; Bachir, R.; Choukchou-Braham, N. Synthesis of Bis-Hydrazine Using Heterogeneous Catalysis. Chem. Proc. 2022, 8, 88. https://doi.org/10.3390/ecsoc-25-11706
Medjahed N, Kibou Z, Berrichi A, Bachir R, Choukchou-Braham N. Synthesis of Bis-Hydrazine Using Heterogeneous Catalysis. Chemistry Proceedings. 2022; 8(1):88. https://doi.org/10.3390/ecsoc-25-11706
Chicago/Turabian StyleMedjahed, Nassima, Zahira Kibou, Amina Berrichi, Redouane Bachir, and Nourredine Choukchou-Braham. 2022. "Synthesis of Bis-Hydrazine Using Heterogeneous Catalysis" Chemistry Proceedings 8, no. 1: 88. https://doi.org/10.3390/ecsoc-25-11706
APA StyleMedjahed, N., Kibou, Z., Berrichi, A., Bachir, R., & Choukchou-Braham, N. (2022). Synthesis of Bis-Hydrazine Using Heterogeneous Catalysis. Chemistry Proceedings, 8(1), 88. https://doi.org/10.3390/ecsoc-25-11706





