Abstract
Pyrazoles and their derivatives have attracted particular attention because they have a wide variety of biological activities, and recently, we found that 4,4’-(arylmethylene)-bis-(1-phenyl-3-methyl-1H-pyrazole-5-ols) have good leishmanicidal activity against promastigotes of Leishmania mexicana. 4-Arylidenepyrazolone derivatives also have antiparasitic activity and are intermediates in the synthesis of these bispirazoles that are formed by the equimolar reaction of 3-methyl-1-phenyl-2-pyrazoline-5-one with aromatic aldehydes. In order to obtain new compounds with potential leishmanicidal activity, we attempted to synthesize several 4-arylallylidenepyrazolone derivatives through the reaction of pyrazol-3-one with different cinnamaldehydes. We report here the study of the synthesis of some 4-arylallylidenepyrazolone derivatives from the reaction between 5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one and 4-nitrocinnamaldehyde. We found that L-proline and FeCl3 were the best catalysts, and we also observed a solvent effect in the reaction. Our preliminary results indicate that aprotic solvents favor the formation of the 2Z isomer instead of the 2E isomer.
1. Introduction
In heterocyclic chemistry, pyrazolones correspond to a type of important molecules with a wide range of reported biological activities. Currently, several drugs on the market possess the pyrazolone ring as a key structure, and its presence confers a wide range of properties such as anti-inflammatory, antiviral, antibacterial, antifungal, and antiparasitic activities, among others [1]. Derivatives of 2,4-dihydro-3H-pyrazol-3-one include edaravone and 4,4′-(arylmethylene)bis(1-phenyl-3-methyl-1H-pyrazol-5-ol). Edaravone, (3-methyl-1-phenyl-2-pyrazoline-5-one) (1) is a free-radical scavenger that is used as a treatment for cardiovascular diseases [2], as a neuroprotectant [3], and has been approved to treat amyotrophic lateral sclerosis (ALS) [4]. 4,4′-(Arylmethylene)bis(1-phenyl-3-methyl-1H-pyrazol-5-ol) derivatives have several biological activities and have been used as antiviral, antibacterial, anticancer and anti-inflammatory [5,6,7,8,9] compounds. Additionally, we recently found good anti-trypanosomatid activity of these compounds, which will be reported elsewhere.
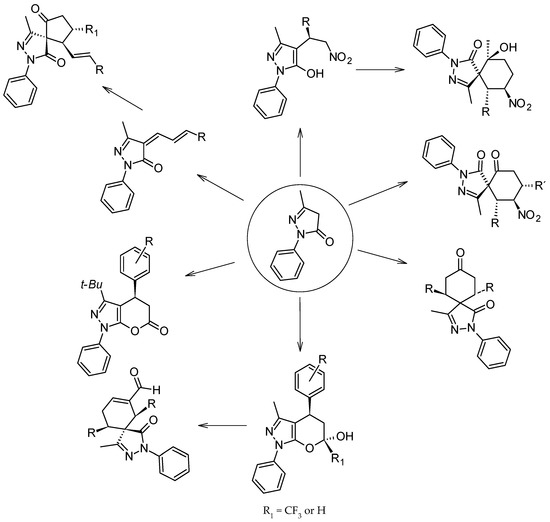
Other derivatives of biological interest are the products obtained by nucleophilic addition of edaravone 1 to various acceptors (Scheme 1) [10]. When these acceptors are nitroalkenes, the corresponding Michael addition products are obtained, and the use of an asymmetric catalyst yields chiral pyrazol-3-ol derivatives [11,12,13]. A second Michael reaction with cinnamaldehyde allows the production of spiro[cyclohexanone-pyrazolones] in moderate to high yields with moderate to good diastereoselectivities and excellent enantioselectivities [14]. Similar spiropyrazolones can also be obtained when pyrazolone 1 is treated with dibenzalacetones in the presence of an amine catalyst to afford spiro[cyclohexanone-pyrazolones] derivatives with high yields and high stereoselectivities. These spiro-derivatives are obtained by cascade [5 + 1] double Michael reactions [15].
Scheme 1.
Different pyrazolone derivatives.
The equimolar reaction of 1 with α,β-unsaturated trifluoromethyl ketones affords trifluoromethylated pyranopyrazoles after a tandem Michael addition/aromatization/cyclization reaction [16]. The same structure is obtained when 1 reacts with trans-cinnamaldehydes using diaryl prolinol silyl ethers as catalyst [17,18]. However, the reaction with a second molecule of cinnamaldehyde, using the same catalyst, affords spyropyrazolones through a Michael–Aldol cascade reaction [17,19,20]. The same reaction under NHC-catalyzed conditions, using chiral triazolium salt and Na2CO3 as base, results in the enantioselective synthesis of dihydropyranone-fused pyrazole [21].
In all the above reactions, the addition of 1 occurs by a 1,4 addition; however, it is also possible, in the case of reactions with cinnamaldehydes, to have a 1,2 addition, which affords 4-arylallylidenepyrazolone derivatives after a Knoevenagel condensation [21,22,23]. These condensates are similar in structure to 4-arylidenepyrazolones, which are intermediates in the synthesis of 4,4′-(arylmethylene)bis(1-phenyl-3-methyl-1H-pyrazol-5-ols), and are formed by the equimolar reaction of 3-methyl-1-phenyl-2-pyrazoline-5-one with aromatic aldehydes [24], and among the different biological activities reported for 4-arylidenepyrazolones, they also present antiparasitic activity [25].
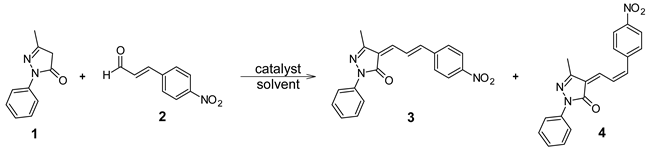
Our laboratory has worked on the synthesis of heterocyclic compounds with possible antiparasitic activity [26,27], and in view of the great structural similarity that 4-arylallylidenepyrazolones present versus 4-arylidenepyrazolones, we attempted to synthesize several 4-arylallylidenepyrazolone derivatives through the reaction of 1 with different cinnamaldehydes. We report here the initial study of the synthesis of 4-arylallylidenepyrazolones, using the reaction between 3-methyl-1-phenyl-2-pyrazoline-5-one (1) and 4-nitrocinnamaldehyde (2) as a model, as well as different catalysts and solvent conditions.
2. Methods
2.1. General
All solvents and reagents used in the investigation were from Sigma-Aldrich and were used without further purification. Melting points were determined on a Büchi Melting Point M-560 apparatus. The 1H- and 13C-NMR spectra were recorded at 298 K on a BRUKER Ascend 500 MHz spectrometer using CDCl3 as the solvent. The photoisomerization was evaluated on an Oxford Instruments Pulsar benchtop NMR 60 MHz Spectrometer. Chemical shifts are expressed in ppm with TMS as an internal reference (TMS, δ = 0 ppm) for protons. The IR spectra were recorded with a VARIAN 660-IR/FT-IR spectrometer (4000–400 cm−1). Reactions were monitored by TLC on silica gel using chloroform and 1:4 ethyl acetate/hexane as the mobile phase, and compounds were visualized by UV lamp at 254 nm. Kinetic data were calculated in GraphPad Prism (GraphPad Software, San Diego, CA, USA).
2.2. General Procedure for the Synthesis of (4Z)-5-Methyl-4-[3-(4-nitrophenyl)-allylidene]-2-phenyl-2,4-dihydro-3H-pyrazol-3-one 3 and 4
To an equimolar solution of 4-nitrocinnamaldehyde (0.564 mmol) and edaravone (0.564 mmol) in 2.0 mL of solvent, 0.0564 mmol of catalyst were added and the mixture was stirred until the reaction was complete (Table 1). The solvent was evaporated under reduced pressure and the residue was dissolved with 2.0 mL of ethanol. Finally, with constant stirring, water was added to obtain 50% EtOH and the mixture was stored at 4 °C. The precipitates formed were collected by filtration, rinsed with cool 50% EtOH and dried under vacuum. After column chromatography on silica gel using chloroform as the eluent, 3 and 4 were obtained as pure products.

Table 1.
Optimization of the Reaction Conditions.
(4Z)-5-methyl-4-[(2E)-3-(4-nitrophenyl)-allylidene]-2-phenyl-2,4-dihydro-3H-pyrazol-3-one (3): Brown powder; mp: 216 °C–218 °C; 1H-NMR (500 MHz, CDCl3) δ 2.30 (s, 3H), 7.20 (dd, J = 11.6, 0.8 Hz, 1H), 7.20 (tt, J = 7.4, 1.1 Hz, 1H), 7.24 (d, J = 15.7 Hz, 1H), 7.42 (dd, J = 8.5, 7.5 Hz, 2H), 7.78 (d, J = 8.8 Hz, 2H), 7.94 (dd, J = 8.8, 1.1 Hz, 2H), 8.26 (d, J = 8.8 Hz, 2H), 8.74 (dd, J = 15.7, 11.5 Hz, 1H); 13C-NMR (126 MHz, CDCl3) δ 12.9, 118.8, 124.4, 125.1, 127.0, 128.0, 129.0, 138.3, 141.6, 142.5, 145.1, 148.5, 149.5, 162.8. FTIR (cm−1): 1687, 1614, 1592, 1511, 1489, 1337, 1131, 977.3, 758.0.
(4Z)-5-methyl-4-[(2Z)-3-(4-nitrophenyl)-allylidene]-2-phenyl-2,4-dihydro-3H-pyrazol-3-one (4): Brown powder; mp: 217 °C–219 °C; 1H-NMR (500 MHz, CDCl3) δ 2.58 (s, 3H), 7.19 (tt, J = 7.4, 1.1 Hz, 1H), 7.33 (d, J = 13.5 Hz, 1H), 7.42 (dd, J = 8.5, 7.5 Hz, 1H), 7.54 (dd, J = 12.3, 0.8 Hz, 1H), 7.58 (dd, J = 13.4, 12.4 Hz, 1H), 7.72 (d, J = 8.8 Hz, 2H), 7.94 (dd, J = 8.7, 1.0 Hz, 2H), 8.29 (d, J = 8.8 Hz, 2H); 13C-NMR (126 MHz, CDCl3) δ 12.9, 118.7, 118.8, 124.4, 125.1, 127.1, 128.1, 128.7, 129.0, 138.3, 141.6, 142.5, 145.1, 149.5, 162.9. FTIR (cm−1): 1671, 1613, 1523, 1339, 1155, 1000, 838.3, 761.1.
2.3. Photoisomerization of 4
A solution of 4 in CDCl3 was irradiated with a blue LED light, and the isomerization was followed by 1H-NMR. Data were analyzed with the statistical software GraphPad Prism.
3. Results and Discussions
4-Arylallylidenepyrazolones were synthesized using NaOAc as a catalyst, and acetic anhydride [23] and acetic acid [28] as the solvents under refluxing conditions. The uncatalyzed reaction under reflux conditions has also been reported [22]; however, the best yield was reported in the uncatalyzed reaction at room temperature using THF as solvent after 24 h of reaction [21].
We began our studies with 3-methyl-1-phenyl-2-pyrazolin-5-one (1) and 4-nitrocinnamaldehyde (2) as test substrates. When the reaction was carried out without the catalyst in THF, a 36% yield of product 3 was obtained after 24 h of reaction (Table 1, entry 1). However, when the reaction was performed with DABCO (10 mol%) as the catalyst at room temperature in EtOH (entry 2), the unsaturated pyrazolone 3 was obtained in 73% yield (based on 1H-NMR spectroscopy) after 60 min of reaction. In addition, in both reactions the pyrazolone 4 was also obtained with 6% and 5% yields, respectively, and no evidence of the formation of a Michael adduct was observed.
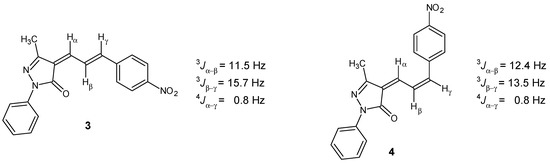
Spectroscopic analysis of 3 and 4 revealed that both compounds are the result of the Knoevenagel condensation of pyrazolone 1 to 4-nitrocinnamaldehyde; however, to our surprise, pyrazolone 4 exhibited a cis configuration at C2 instead of the trans configuration observed in 2 (Scheme 2). The 1H-NMR spectrum of 3 is in agreement with the expected structure (Scheme 2). Hβ appears at 8.74 ppm and shows two couplings, H-H, 3Jβ-α = 11.5 and Hz and 3Jβ-γ = 15.7 Hz, while Hα and Hγ appear at 7.20 and 7.24 ppm, respectively. These couplings show that the obtained isomer corresponds to 2E. On the other hand, in the case of pyrazolone 4, Hβ appears at 7.58 ppm and shows two couplings, H-H, 3Jβ-α = 12.4 Hz and 3Jβ-γ = 13.5 Hz. This proton shows an upfield shift of 1.16 ppm, possibly due to loss of conjugation owing to the conformation of the 2Z isomer.
Scheme 2.
Coupling constant H-H of the unsaturated system of 3 and 4.
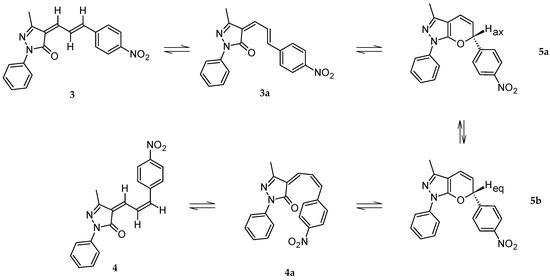
The possible mechanism for the formation of the 4-arylallylidenepyrazolone 4 is depicted in Scheme 3. The Knoevenagel condensation of 1 with 2 affords 3 as the only regioisomer, as confirmed by 1D-NOESY. The 4-arylallylidenepyrazolone 3 is a 1-oxatriene that undergoes a reversible pericyclic oxa-6π-electrocyclization process until the 2H-pyran structure 5a (valence isomerization) is obtained [29]. In 5a the Hγ is in an axial position, while in their conformer 5b, it would be in an equatorial position. A second valence isomerization of 5b allows the production of the pyrazolone 4. Generally, compounds that have a 2H-pyran ring attached to an aromatic ring are stable enough to remain in the cyclic form (i.e., 2H-chromenes), otherwise, they tend to be unstable and prefer the opened isomeric form [30]. Likewise, simpler dienones, which could adopt a stable planar conformation, existed in the opened form. This is favored in the case of the 4-arylallylidenepyrazolones since they present an extended π-system [31]. Furthermore, no spectroscopic evidence of the formation of 5a or 5b was observed.
Scheme 3.
Possible mechanism of the cis-trans-cis isomerization of 3 to 4.
A kinetic study of the photoisomerization of a solution of 4 in CDCl3 was carried out, in which the solution was irradiated with a blue LED light and evaluated by 1H-NMR, observing that the half-life of 4 was 25 min, and at equilibrium 98.6% pyrazolone 3 was formed (Figure 1).
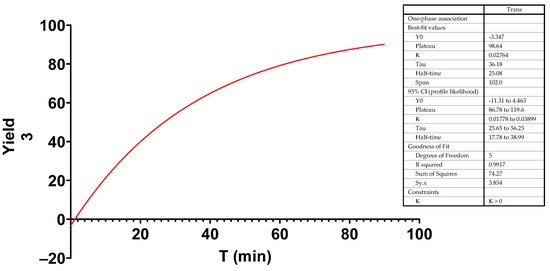
Figure 1.
Yield of formation of 3 in CDCl3 after photoisomerization of 4 at different times of irradiation.
Different solvents were screened (entries 2–7) and it turned out that the highest total yield was obtained in EtOH. In all the evaluated solvents, both diastereomeric pyrazolones 3 and 4 were obtained, except in THF and acetonitrile, where only isomer 3 was formed, both with 75% yield. In the other solvents, the highest stereoselectivity was obtained in EtOH with a 14.6:1 diastereomeric ratio of 3 and 4. Additional studies on the effect of catalyst loading have shown that the best yield was observed with 10 mol%, yielding pyrazolones 3 and 4 with 78% yield (Table 1, entries 2, 8–10). Finally, using EtOH as a solvent, the effect of the catalyst on the reaction was evaluated (entries 11–20). In all cases, pyrazolone 3 was the main product. The uncatalyzed reaction produced a 55% yield with no stereoselectivity (entry 11), while FeCl3 and L-proline were the best catalysts with 84% and 85% of total yields, respectively.
4. Conclusions
The synthesis of the (4Z)-5-methyl-4-[3-(4-nitrophenyl)-allylidene]-2-phenyl-2,4-dihydro-3H-pyrazol-3-one isomers 3 and 4 can be easily carried out through the Knoevenagel reaction between edaravone (1) and 4-nitrocinnamaldehyde (2). Both pyrazolones were formed in most of the solvents used; however, it can be observed that in protic solvents the diastereomeric ratio favored the pirazolone 3 (2E), while in aprotic and nonpolar solvents, the formation of the pirazolone 4 (2Z) was increased. In THF and acetonitrile the synthesis of 4 was stereospecific.
Finally, L-proline and FeCl3 were the best catalysts, and with both, the diastereotopic ratio obtained was about 4:1; however, using DABCO in EtOH, good yield and a high diastereotopic ratio were obtained, which favored the synthesis of pyrazolone 3.
Author Contributions
Conceptualization, E.A.-L., J.C.R.-B. and J.H.-M.; methodology, E.A.-L., J.C.R.-B. and J.H.-M.; validation, E.A.-L., J.C.R.-B. and J.H.-M.; formal analysis, J.H.-M.; investigation, E.A.-L., J.C.R.-B. and J.H.-M.; resources, E.A.-L., J.C.R.-B. and J.H.-M.; data curation, E.A.-L., and J.H.-M.; writing—original draft preparation, E.A.-L. and J.H.-M.; writing—review and editing, E.A.-L. and J.H.-M.; visualization, E.A.-L., J.C.R.-B. and J.H.-M.; supervision, J.H.-M.; project administration, J.H.-M.; funding acquisition, J.H.-M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Acknowledgments
This study was funded by Universidad UTE and Universidad Técnica Particular de Loja (UTPL).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhao, Z.; Dai, X.; Li, C.; Wang, X.; Tian, J.; Feng, Y.; Xie, J.; Ma, C.; Nie, Z.; Fan, P.; et al. Pyrazolone structural motif in medicinal chemistry: Retrospect and prospect. Eur. J. Med. Chem. 2020, 186, 111893. [Google Scholar] [CrossRef]
- Higashi, Y.; Jitsuiki, D.; Chayama, K.; Yoshizumi, M. Edaravone (3-methyl-1-phenyl-2-pyrazolin-5-one), a novel free radical scavenger, for treatment of cardiovascular diseases. Recent Pat. Cardiovasc. Drug Discov. 2006, 1, 85–93. [Google Scholar] [CrossRef]
- Yuan, W.J.; Yasuhara, T.; Shingo, T.; Muraoka, K.; Agari, T.; Kameda, M.; Uozumi, T.; Tajiri, N.; Morimoto, T.; Jing, M.; et al. Neuroprotective effects of edaravone-administration on 6-OHDA-treated dopaminergic neurons. BMC Neurosci. 2008, 9, 75. [Google Scholar] [CrossRef]
- Bailly, C.; Hecquet, P.E.; Kouach, M.; Thuru, X.; Goossens, J.F. Chemical reactivity and uses of 1-phenyl-3-methyl-5-pyrazolone (PMP), also known as edaravone. Bioorg. Med. Chem. 2020, 28, 115463. [Google Scholar] [CrossRef]
- Sujatha, K.; Shanthi, G.; Selvam, N.P.; Manoharan, S.; Perumal, P.T.; Rajendran, M. Synthesis and antiviral activity of 4,4′-(arylmethylene)bis(1H-pyrazol-5-ols) against peste des petits ruminant virus (PPRV). Bioorg. Med. Chem. Lett. 2009, 19, 4501–4503. [Google Scholar] [CrossRef]
- Bhavanarushi, S.; Kanakaiah, V.; Bharath, G.; Gangagnirao, A.; Vatsala Rani, J. Synthesis and antibacterial activity of 4,4′-(aryl or alkyl methylene)-bis(1H-pyrazol-5-ol) derivatives. Med. Chem. Res. 2014, 23, 158–167. [Google Scholar] [CrossRef]
- Mahajan, P.S.; Nikam, M.D.; Khedkar, V.; Jha, P.; Badadhe, P.V.; Gilla, C.H. An Organocatalyzed Efficient One-pot Synthesis, Biological Evaluation, and Molecular Docking Studies of 4,4′-(Arylmethylene)bis-(3-methyl-1-phenyl-1H-pyrazol-5-ols). J. Heterocycl. Chem. 2017, 54, 1109–1120. [Google Scholar] [CrossRef]
- Diwan, F.; Shaikh, M.; Farooqui, M. Lemon juice catalyzed efficient one-pot synthesis, antioxidant and antimicrobial evaluation of bispyrazolyl methanes. Chem. Biol. Interface 2018, 8, 255–268. [Google Scholar]
- Cadena-Cruz, J.E.; Guamán-Ortiz, L.M.; Romero-Benavides, J.C.; Bailon-Moscoso, N.; Murillo-Sotomayor, K.E.; Ortiz-Guamán, N.V.; Heredia-Moya, J. Synthesis of 4,4′-(arylmethylene)bis(3-methyl-1-phenyl-1H-pyrazol-5-ols) and evaluation of their antioxidant and anticancer activities. BMC Chem. 2021, 15, 38. [Google Scholar] [CrossRef]
- Chauhan, P.; Mahajan, S.; Enders, D. Asymmetric synthesis of pyrazoles and pyrazolones employing the reactivity of pyrazolin-5-one derivatives. Chem. Commun. 2015, 51, 12890–12907. [Google Scholar] [CrossRef]
- Li, J.-H.; Du, D.-M. Squaramide-catalysed enantioselective Michael addition of pyrazolin-5-ones to nitroalkenes. Org. Biomol. Chem. 2013, 11, 6215. [Google Scholar] [CrossRef]
- Hack, D.; Chauhan, P.; Deckers, K.; Mizutani, Y.; Raabe, G.; Enders, D. Combining silver- and organocatalysis: An enantioselective sequential catalytic approach towards pyrano-annulated pyrazoles. Chem. Commun. 2015, 51, 2266–2269. [Google Scholar] [CrossRef]
- Amireddy, M.; Chen, K. Organocatalytic one-pot asymmetric synthesis of functionalized spiropyrazolones via a Michael-aldol sequential reaction. RSC Adv. 2016, 6, 77474–77480. [Google Scholar] [CrossRef]
- Li, J.-H.; Cui, Z.-H.; Du, D.-M. Diastereo- and enantioselective construction of cyclohexanone-fused spirospyrazolones containing four consecutive stereocenters through asymmetric sequential reactions. Org. Chem. Front. 2016, 3, 1087–1090. [Google Scholar] [CrossRef]
- Wu, B.; Chen, J.; Li, M.Q.; Zhang, J.X.; Xu, X.P.; Ji, S.J.; Wang, X.W. Highly enantioselective synthesis of spiro[cyclohexanone-oxindoles] and spiro[cyclohexanone-pyrazolones] by asymmetric cascade [5+1] double Michael reactions. Eur. J. Org. Chem. 2012, 1318–1327. [Google Scholar] [CrossRef]
- Wang, J.; Huang, G.B.; Yang, L.J.; Li, F.; Nie, J.; Ma, J.A. Tandem stereoselective synthesis of new trifluoromethylated pyranopyrazoles. J. Fluor. Chem. 2015, 171, 27–35. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, S.; Wang, S.; Fang, K.; Dong, G.; Liu, N.; Miao, Z.; Yao, J.; Li, J.; Zhang, W.; et al. Divergent cascade construction of skeletally diverse “privileged” pyrazole-derived molecular architectures. Eur. J. Org. Chem. 2015, 2015, 2030–2037. [Google Scholar] [CrossRef]
- Wu, S.; Li, Y.; Xu, G.; Chen, S.; Zhang, Y.; Liu, N.; Dong, G.; Miao, C.; Su, H.; Zhang, W.; et al. Novel spiropyrazolone antitumor scaffold with potent activity: Design, synthesis and structure-activity relationship. Eur. J. Med. Chem. 2016, 115, 141–147. [Google Scholar] [CrossRef]
- Companyó, X.; Zea, A.; Alba, A.N.R.; Mazzanti, A.; Moyano, A.; Rios, R. Organocatalytic synthesis of spiro compounds via a cascade Michael-Michael-aldol reaction. Chem. Commun. 2010, 46, 6953–6955. [Google Scholar] [CrossRef]
- Alba, A.-N.R.; Zea, A.; Valero, G.; Calbet, T.; Font-Bardía, M.; Mazzanti, A.; Moyano, A.; Rios, R. Highly Stereoselective Synthesis of Spiropyrazolones. Eur. J. Org. Chem. 2011, 2011, 1318–1325. [Google Scholar] [CrossRef]
- Yetra, S.R.; Mondal, S.; Suresh, E.; Biju, A.T. Enantioselective synthesis of functionalized pyrazoles by NHC-catalyzed reaction of pyrazolones with α,β-unsaturated aldehydes. Org. Lett. 2015, 17, 1417–1420. [Google Scholar] [CrossRef]
- Huang, H.; Yu, Y.; Gao, Z.; Zhang, Y.; Li, C.; Xu, X.; Jin, H.; Yan, W.; Ma, R.; Zhu, J.; et al. Discovery and Optimization of 1,3,4-Trisubstituted-pyrazolone Derivatives as Novel, Potent, and Nonsteroidal Farnesoid X Receptor (FXR) Selective Antagonists. J. Med. Chem. 2012, 55, 7037–7053. [Google Scholar] [CrossRef]
- Szukalski, A.; Jędrzejewska, B.; Krawczyk, P.; Bajorek, A. An optical modulator on the pyrazolone-based bi-component system. Dye. Pigment. 2020, 172, 107805. [Google Scholar] [CrossRef]
- Ma, R.; Zhu, J.; Liu, J.; Chen, L.; Shen, X.; Jiang, H.; Li, J. Microwave-Assisted One-Pot Synthesis of Pyrazolone Derivatives under Solvent-Free Conditions. Molecules 2010, 15, 3593–3601. [Google Scholar] [CrossRef]
- Gehrke, S.S. Small Molecules with Anti-trypanosomal and Anti-leishmanial Activity; University of East Anglia: Norwich, UK, 2012. [Google Scholar]
- Rodríguez-Gutiérrez, S.V.; Barreiro-Costa, O.; León, C.D.A.; Heredia-Moya, J. Synthesis and Leishmanicidal Activity of Molecular Hybrids 1,2,3-Triazole-Chalcones. Chem. Proc. 2021, 3, 55. [Google Scholar] [CrossRef]
- Barreiro-Costa, O.; Morales-Noboa, G.; Rojas-Silva, P.; Lara-Barba, E.; Santamaría-Aguirre, J.; Bailón-Moscoso, N.; Romero-Benavides, J.C.; Herrera, A.; Cueva, C.; Ron-Garrido, L.; et al. Synthesis and evaluation of biological activities of bis(spiropyrazolone)cyclopropanes: A potential application against leishmaniasis. Molecules 2021, 26, 4960. [Google Scholar] [CrossRef]
- Li, S.; Wang, L.; Chauhan, P.; Peuronen, A.; Rissanen, K.; Enders, D. Asymmetric Synthesis of Five-Membered Spiropyrazolones via N-Heterocyclic Carbene (NHC)-Catalyzed [3+2] Annulations. Synthesis 2017, 49, 1808–1815. [Google Scholar] [CrossRef][Green Version]
- Krasnaya, Z.A. Dienone ⇆ 2H-pyran valence isomerization. Chem. Heterocycl. Compd. 1999, 35, 1255–1271. [Google Scholar] [CrossRef]
- Tejedor, D.; Delgado-Hernández, S.; Diana-Rivero, R.; Díaz-Díaz, A.; García-Tellado, F. Recent Advances in the Synthesis of 2H-Pyrans. Molecules 2019, 24, 2904. [Google Scholar] [CrossRef]
- Gosink, T.A. Valence isomers. Substituent effects on the equilibrium between 2H-pyrans and cis-dienones. J. Org. Chem. 1974, 39, 1942–1944. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).