Resorufin-Based Colorimetric and Fluorescent Probe for Selective Detection of Mercury (II) †
Abstract
:1. Introduction
2. Previous Research
2.1. Fluorescent Probes for Hg2+ Analysis Based on Ring-Opening Reactions
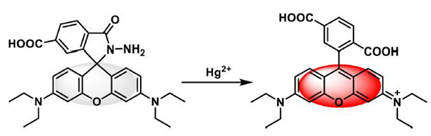
2.2. Fluorescent Probes for Hg2+ Analysis Based on Ring Opening, Followed by Cyclisation
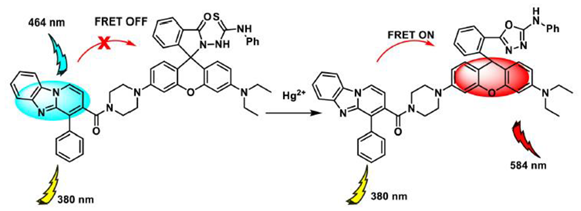
2.3. Fluorescent Probes for Hg2+ Analysis Based on S-Atom Complexation
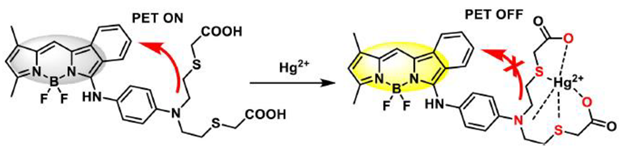
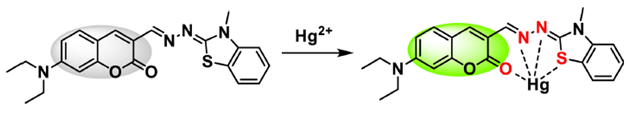
2.4. Fluorescent Probes for Hg2+ Analysis Based on Other Mechanisms
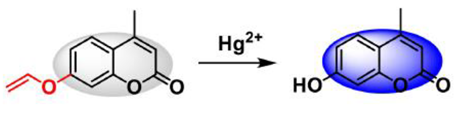
2.5. Fluorescent Probes for Hg2+ Analysis Based on Deprotection of Dithioacetals

3. Hypothesis
4. Materials and Instrumentations
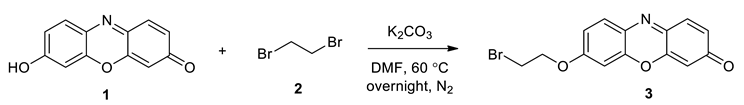
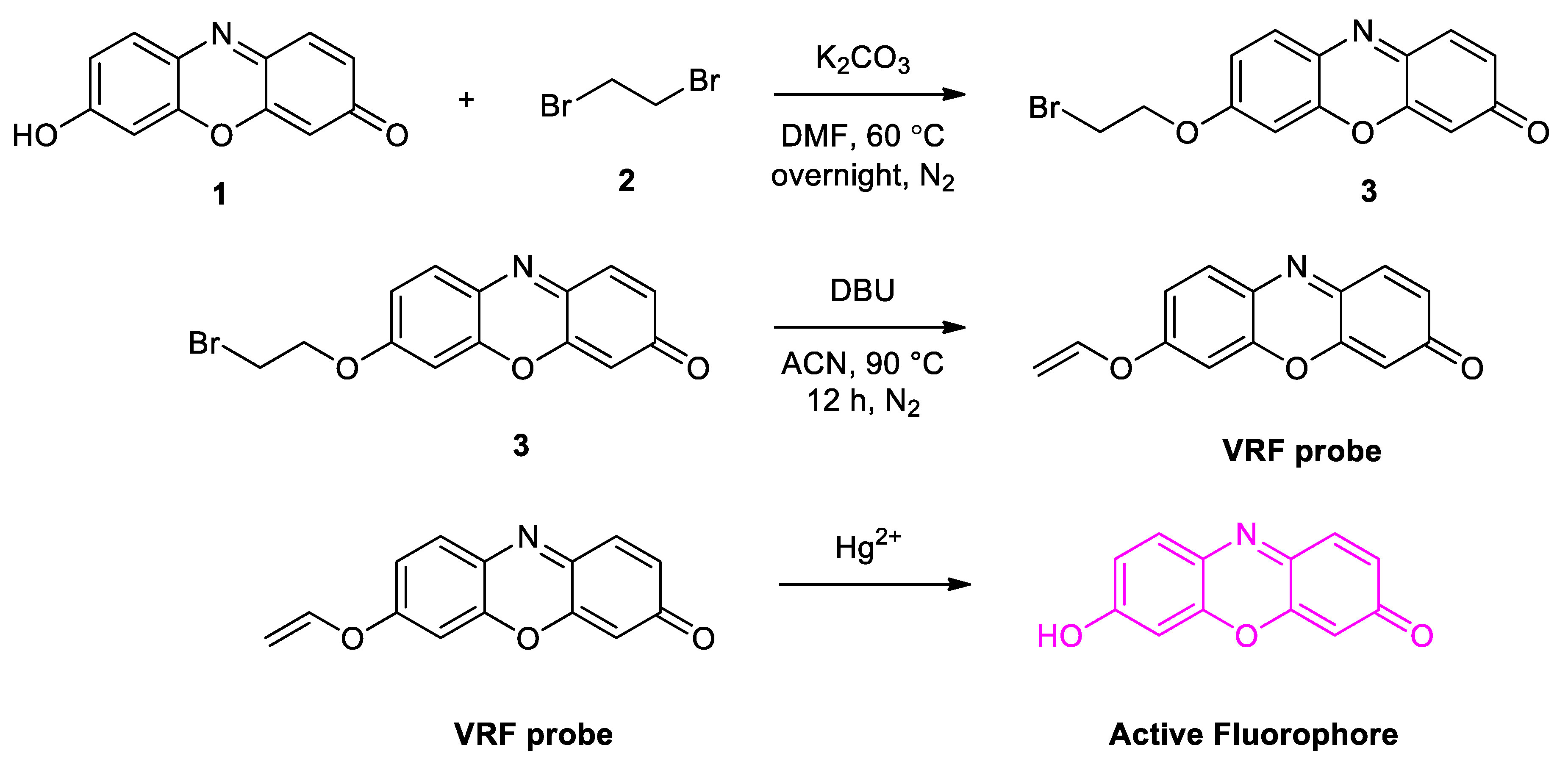
5. Design and Synthesis of Probe
General Procedure for the Synthesis of VRF Probe
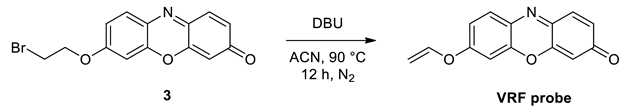
6. Sensing Study
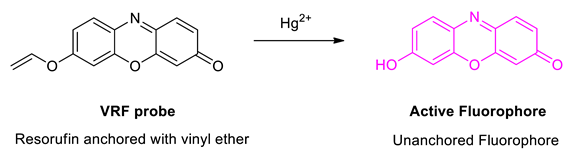
7. Results and Discussion
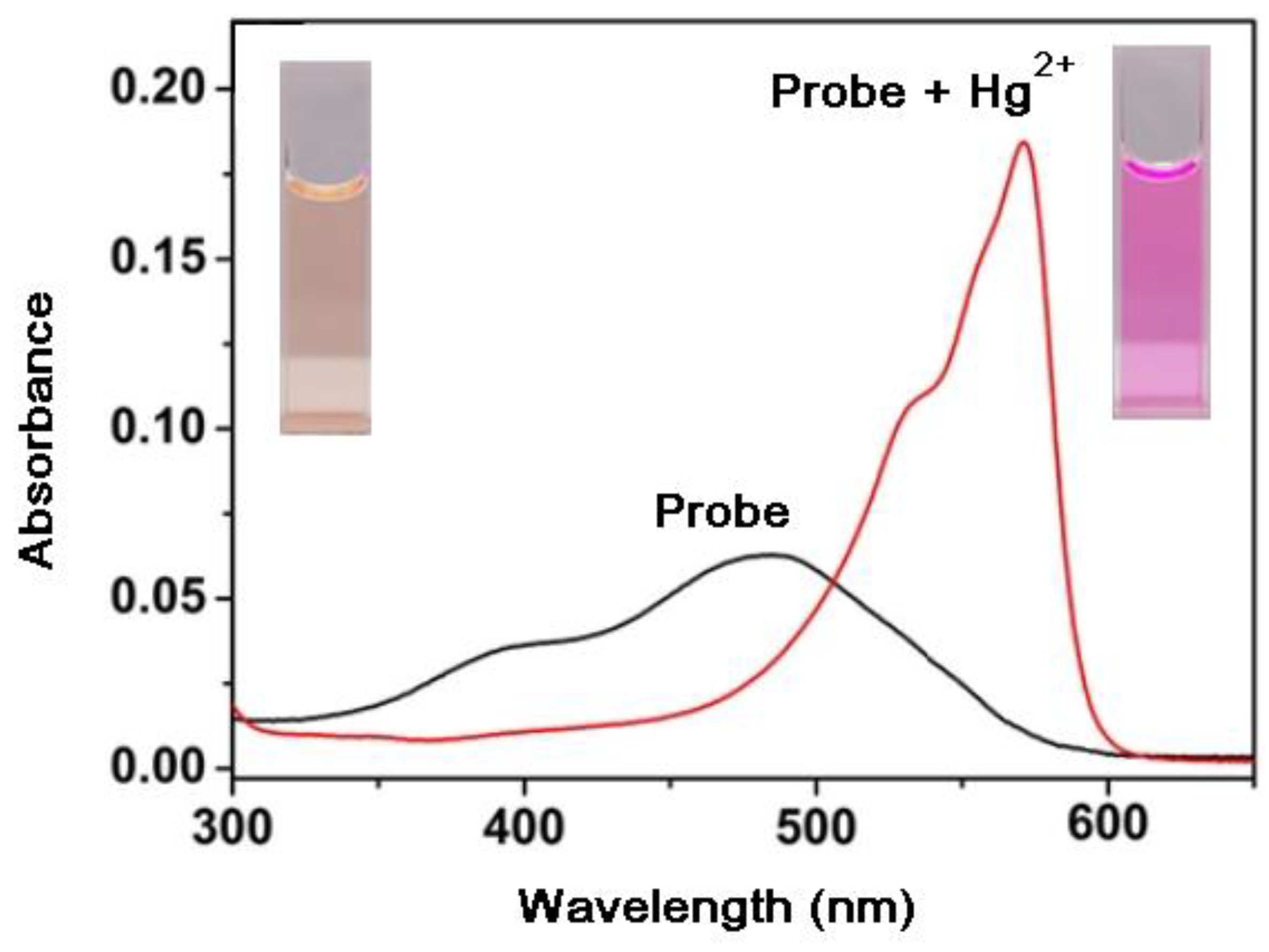
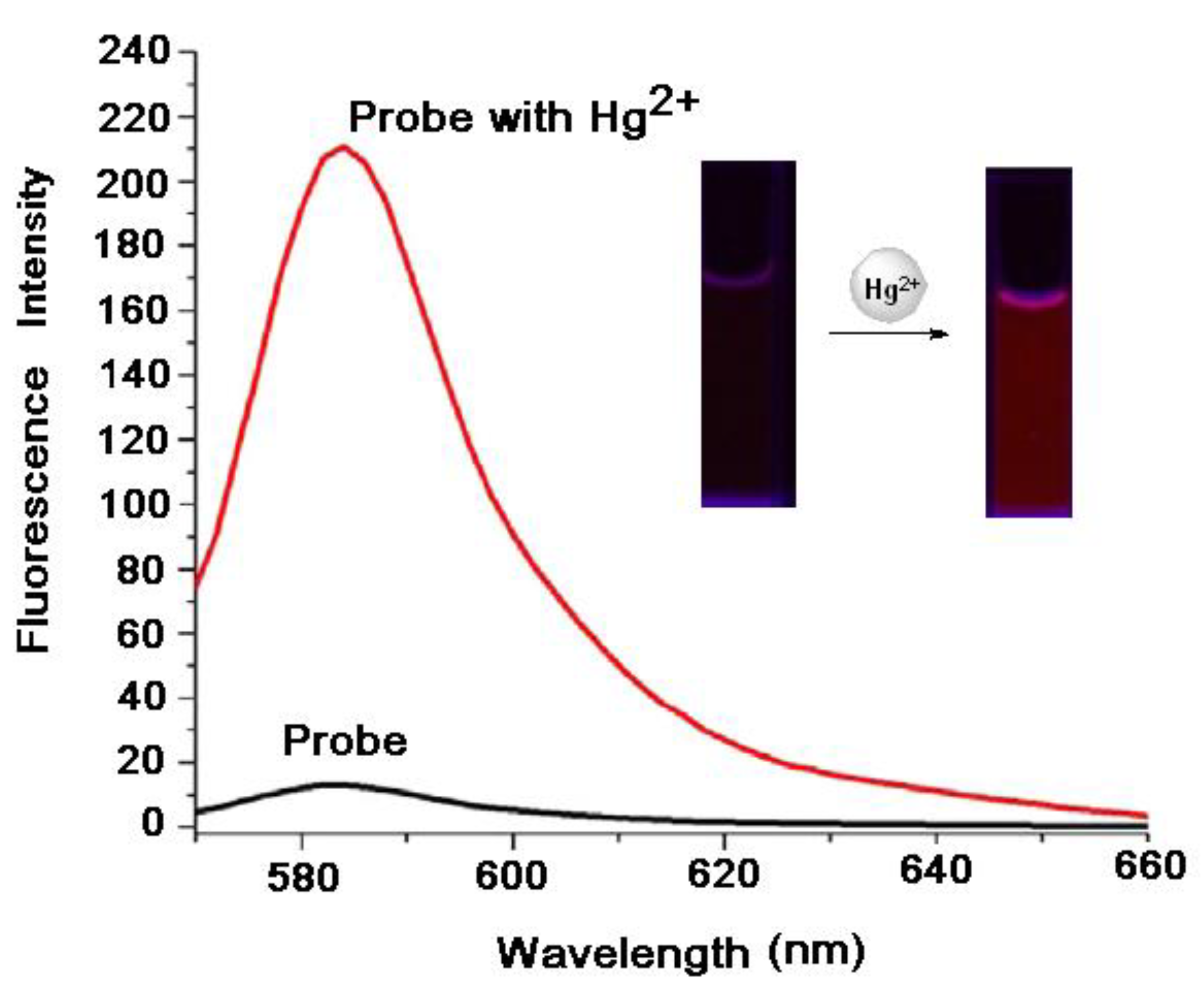
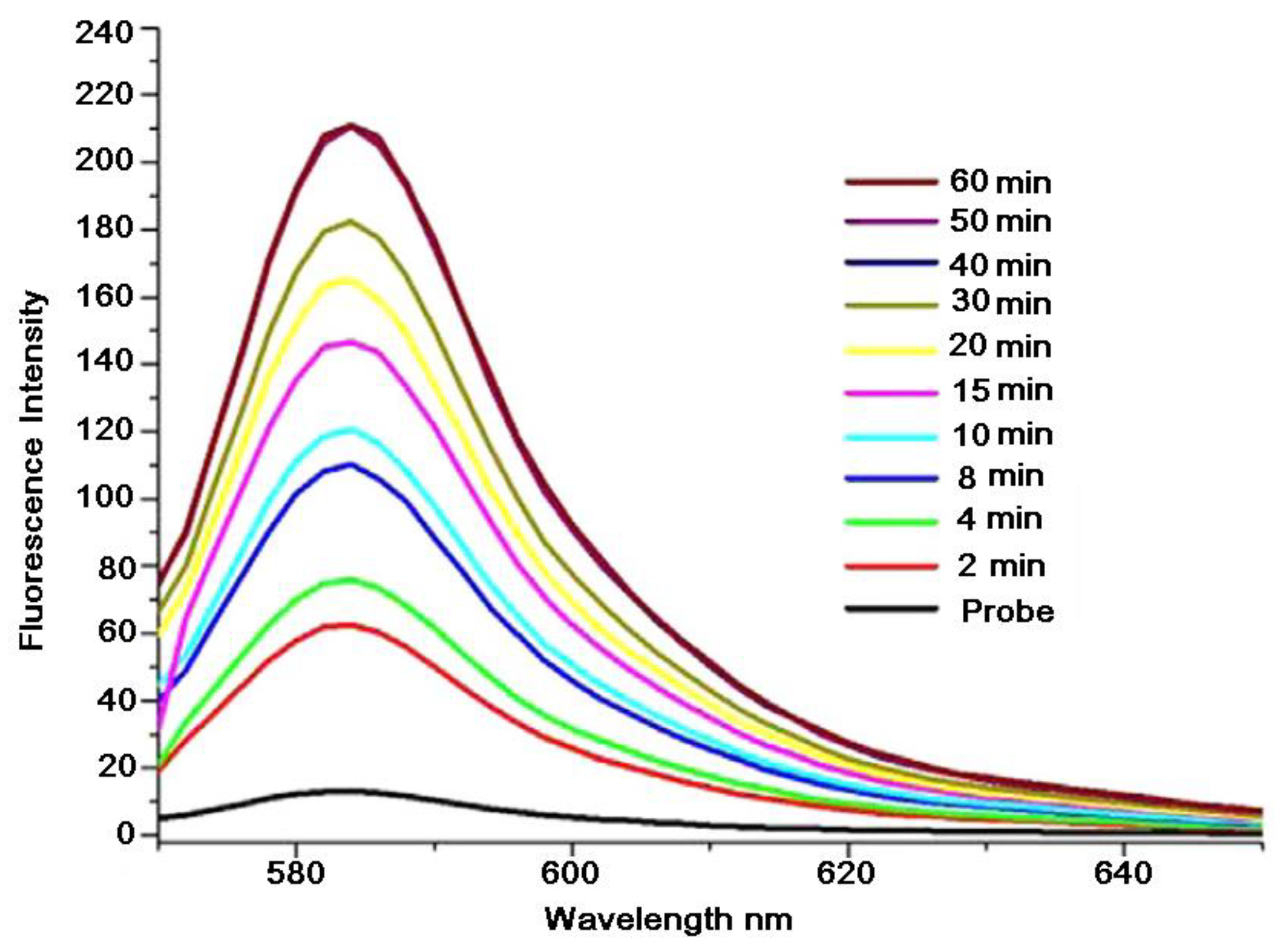
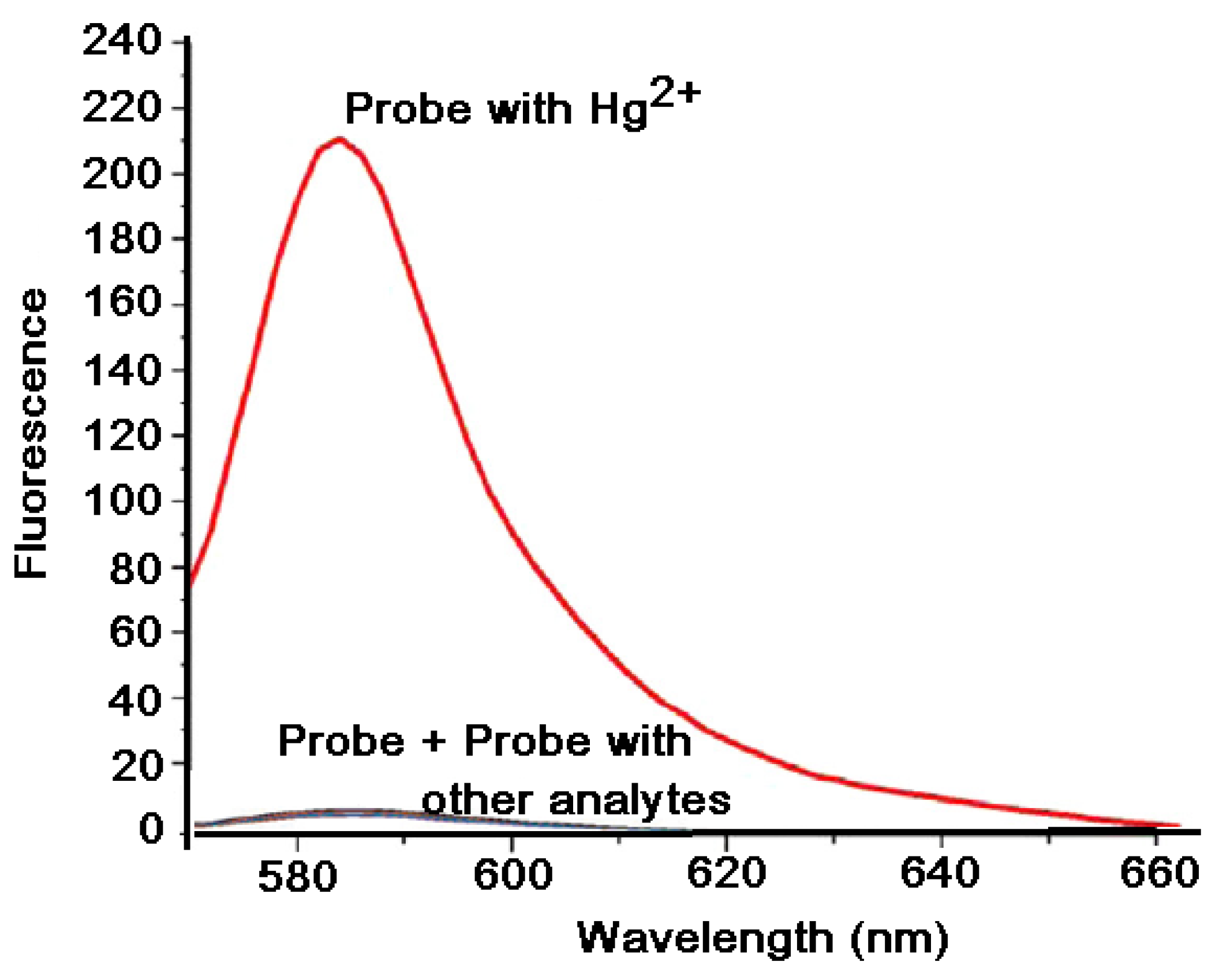
7.1. Effects of Mercury (II) on Absorption and Emission Spectroscopic Properties of VRF Probe
7.2. Response of VRF Probe to Mercury and Other Metal Ions
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nendza, M.; Herbst, T.; Kussatz, C.; Gies, A. Potential for Secondary Poisoning and Biomagnification in Marine Organisms. Chemosphere 1997, 35, 1875–1885. [Google Scholar] [CrossRef]
- Renzoni, A.; Zino, F.; Franchi, E. Mercury Levels along the Food Chain. Environ. Pollut. 1998, 77, 68–72. [Google Scholar] [CrossRef]
- Mason, F.M.M.; Reinfelder, R.P.; Morel, J.R. Bioaccumulation of Mercury and Methylmercury. Water Air Soil Pollut. 1995, 80, 915–921. [Google Scholar] [CrossRef]
- Boening, D.W. Ecological Effects, Transport, and Fate of Mercury: A General Review. Chemosphere 2000, 40, 1335–1351. [Google Scholar] [CrossRef]
- Kim, K.H.; Kabir, E.; Jahan, S.A. A Review on the Distribution of Hg in the Environment and Its Human Health Impacts. J. Hazard. Mater. 2016, 306, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Kraepiel, A.M.L.; Keller, K.; Chin, H.B.; Malcolm, E.G.; Morel, F.M.M. Sources and Variations of Mercury in Tuna. Environ. Sci. Technol. 2003, 37, 5551–5558. [Google Scholar] [CrossRef] [PubMed]
- Burger, J.; Gochfeld, M. Mercury in Canned Tuna: White versus Light and Temporal Variation. Environ. Res. 2004, 96, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Kuwabara, J.S.; Arai, Y.; Topping, B.R.; Pickering, I.J.; George, G.N. Mercury Speciation in Piscivorous Fish from Mining-Impacted Reservoirs. Environ. Sci. Technol. 2007, 41, 2745–2749. [Google Scholar] [CrossRef]
- Kiefer, A.M.; Seney, C.S.; Boyd, E.A.; Smith, C.; Shivdat, D.S.; Matthews, E.; Hull, M.W.; Bridges, C.C.; Castleberry, A. Chemical Analysis of Hg0-Containing Hindu Religious Objects. PLoS ONE 2019, 14, e0226855. [Google Scholar] [CrossRef] [Green Version]
- Guo, Q.; Zhang, Y.; Lin, Z.H.; Cao, Q.Y.; Chen, Y. Fluorescent Norbornene for Sequential Detection of Mercury and Biothiols. Dye Pigment 2020, 172, 107872. [Google Scholar] [CrossRef]
- Li, D.; Li, C.Y.; Li, Y.F.; Li, Z.; Xu, F. Rhodamine-Based Chemodosimeter for Fluorescent Determination of Hg(2+) in 100% Aqueous Solution and in Living Cells. Anal. Chim. Acta 2016, 934, 218–225. [Google Scholar] [CrossRef]
- Ge, Y.; Liu, A.; Ji, R.; Shen, S.; Cao, X. Detection of Hg2þ by a FRET Ratiometric Fluorescent Probe Based on a Novel Pyrido[1,2-a]Benzimidazole-Rhodamine System. Sens. Actuator B Chem. 2017, 251, 410–415. [Google Scholar] [CrossRef]
- Zhou, B.; Qin, S.; Chen, B.; Han, Y. A New BODIPY-Based Fluorescent “Turn-on” Probe for Highly Selective and Rapid Detection of Mercury Ions. Tetrahedron Lett. 2018, 59, 4359–4363. [Google Scholar] [CrossRef]
- Jiao, Y.; Zhou, L.; He, H.; Yin, J.; Duan, C. A New Fluorescent Chemosensor for Recognition of Hg2+ Ions Based on a Coumarin Derivative. Talanta. 2017, 162, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Wang, J.; Shen, J.; Bi, C.; Zhou, H. Coumarin-Based Hg2þ Fluorescent Probe: Synthesis and Turn-on Fluorescence Detection in Neat Aqueous Solution. Sens. Actuator B Chem. 2017, 243, 678–683. [Google Scholar] [CrossRef]
- Zhou, Y.; He, X.; Chen, H.; Wang, Y.; Xiao, S.; Zhang, N.; Li, D.; Zheng, K. An ESIPT/ICT Modulation Based Ratiometric Fluorescent Probe for Sensitive and Selective Sensing Hg2+. Sens. Actuator B Chem. 2017, 247, 626–631. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thakare, M.S.; Patil, D.B.; Kokate, S.V.; Pawar, N.S. Resorufin-Based Colorimetric and Fluorescent Probe for Selective Detection of Mercury (II). Chem. Proc. 2022, 8, 50. https://doi.org/10.3390/ecsoc-25-11779
Thakare MS, Patil DB, Kokate SV, Pawar NS. Resorufin-Based Colorimetric and Fluorescent Probe for Selective Detection of Mercury (II). Chemistry Proceedings. 2022; 8(1):50. https://doi.org/10.3390/ecsoc-25-11779
Chicago/Turabian StyleThakare, Milind Shamrao, Dipak B. Patil, Siddhant V. Kokate, and Nilesh S. Pawar. 2022. "Resorufin-Based Colorimetric and Fluorescent Probe for Selective Detection of Mercury (II)" Chemistry Proceedings 8, no. 1: 50. https://doi.org/10.3390/ecsoc-25-11779
APA StyleThakare, M. S., Patil, D. B., Kokate, S. V., & Pawar, N. S. (2022). Resorufin-Based Colorimetric and Fluorescent Probe for Selective Detection of Mercury (II). Chemistry Proceedings, 8(1), 50. https://doi.org/10.3390/ecsoc-25-11779





