Synthesis, Characterization and Preliminary Antibacterial Evaluation against Staphylococcus aureus of a New 2,4,5-Tri(hetero)arylimidazole Derivative Based on Azaindole Heterocycle †
Abstract
:1. Introduction
2. Materials and Methods
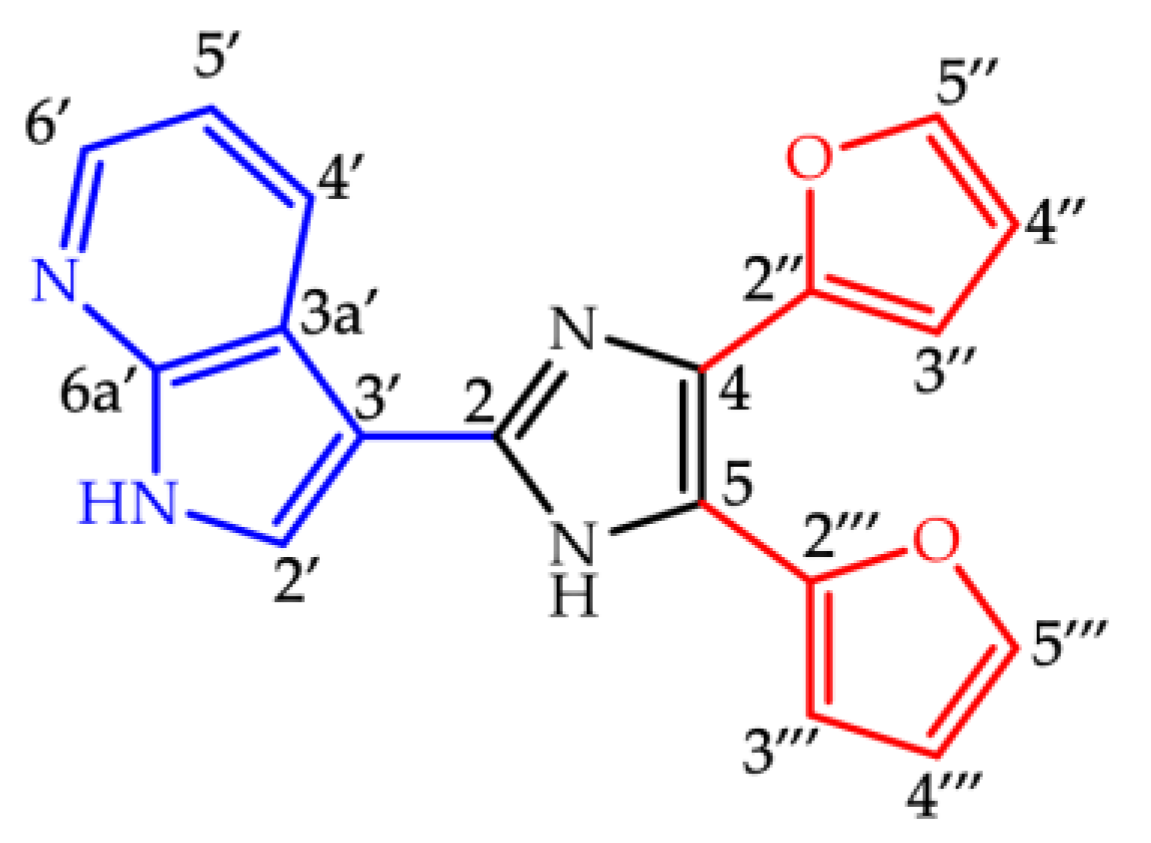
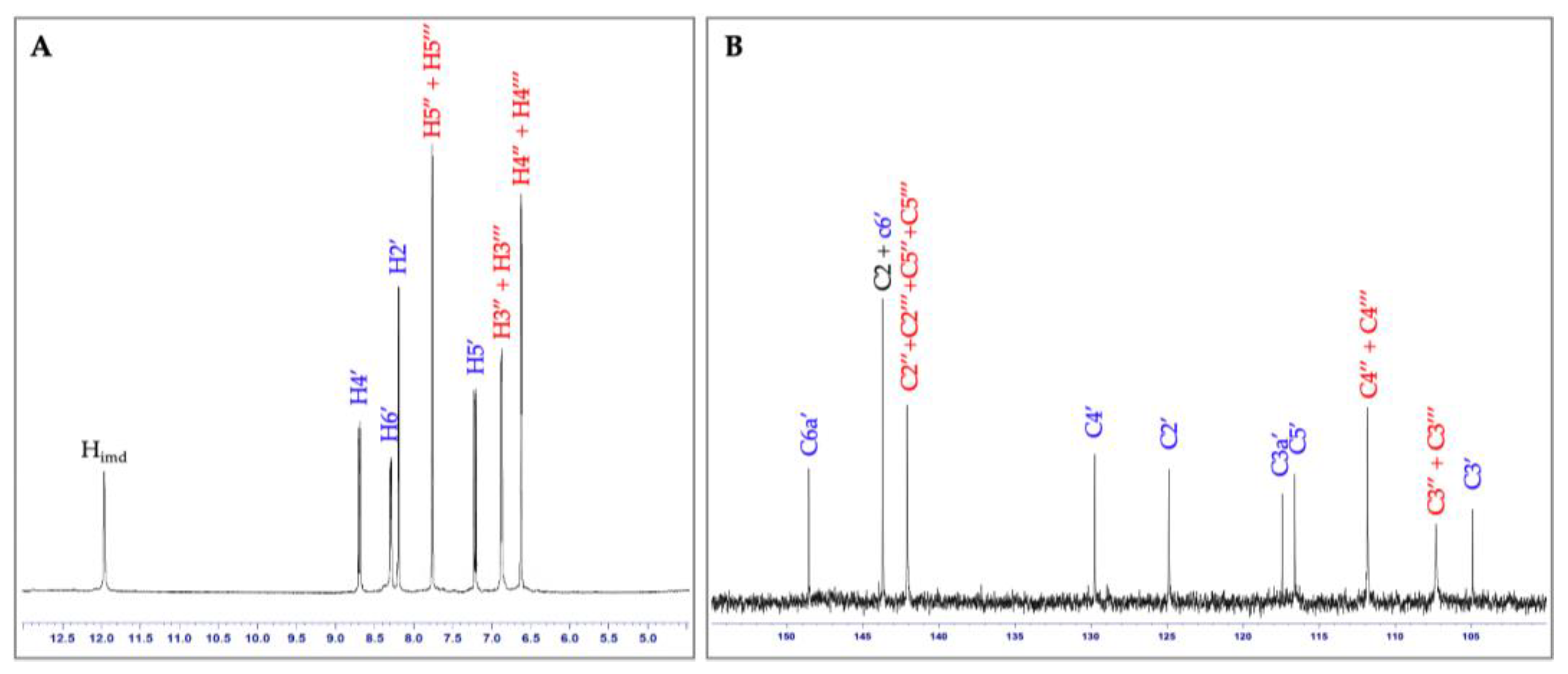
2.1. Synthesis and Spectroscopic Characterization of Imidazole Derivative 3
2.2. Antibacterial Activity of Imidazole Derivative 3
3. Results and Discussion
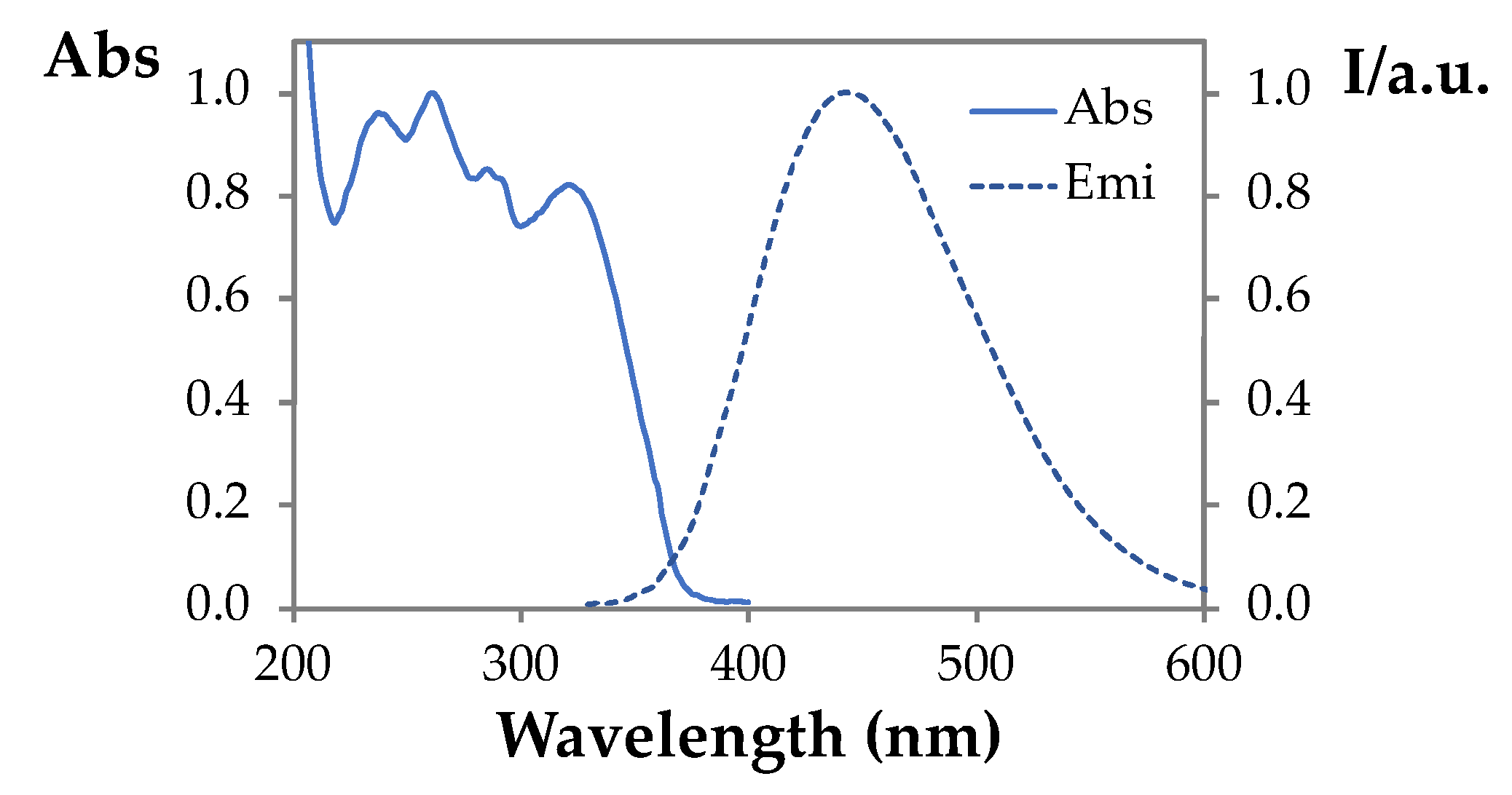
3.1. Synthesis and Spectroscopic Characterization of the Imidazole Derivative 3
3.2. Antibacterial Activity of Imidazole Derivative 3
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferreira, R.C.M.; Costa, S.P.G.; Gonçalves, H.; Belsley, M.; Raposo, M.M.M. Fluorescent phenanthroimidazoles functionalized with heterocyclic spacers: Synthesis, optical chemosensory ability and Two-Photon Absorption (TPA) properties. New J. Chem. 2017, 41, 12866–12878. [Google Scholar] [CrossRef]
- Okda, H.E.; Sayed, S.E.; Ferreira, R.C.M.; Costa, S.P.G.; Raposo, M.M.M.; Martínez-Máñez, R.; Sancenón, F. 4-(4,5-Diphenyl-1H-imidazole-2-yl)-N,N-dimethylaniline-Cu(II) complex, a highly selective probe for glutathione sensing in water-acetonitrile mixtures. Dyes Pigments 2018, 159, 45–48. [Google Scholar] [CrossRef]
- Okda, H.E.; Sayed, S.E.; Ferreira, R.C.M.; Otri, I.; Costa, S.P.G.; Raposo, M.M.M.; Martínez-Máñez, R.; Sancenón, F. A simple and easy-to-prepare imidazole-based probe for the selective chromofluorogenic recognition of biothiols and Cu(II) in aqueous environments. Dyes Pigments 2019, 162, 303–308. [Google Scholar] [CrossRef]
- Moreira, X.; Santos, P.; Faustino, M.A.F.; Raposo, M.M.M.; Costa, S.P.G.; Moura, N.M.M.; Gomes, A.T.P.C.; Almeida, A.; Neves, M.G.P.M.S. An insight into the synthesis of cationic porphyrin-imidazole derivatives and their photodynamic inactivation efficiency against Escherichia coli. Dyes Pigments 2020, 178, 108330. [Google Scholar] [CrossRef]
- Sousa, R.P.C.L.; Figueira, R.B.; Gomes, B.; Costa, S.P.G.; Azenha, M.; Pereira, R.P.C.L.; Raposo, M.M.M. Organic-inorganic hybrid sol-gel materials doped with a fluorescent triarylimidazole derivative. RSC Adv. 2021, 11, 24613–24623. [Google Scholar] [CrossRef]
- Verma, A.; Joshi, S.; Singh, D. Imidazole: Having versatile biological activities. J. Chem. 2013, 2013, 329412. [Google Scholar] [CrossRef]
- Hossain, M. A review on heterocyclic: Synthesis and their application in medicinal chemistry of imidazole moiety. Sci. J. Chem. 2018, 6, 83–94. [Google Scholar] [CrossRef]
- Hu, Y.; Pan, G.; Yang, Z.; Li, T.; Wang, J.; Ansari, M.F.; Hu, C.; Bheemanaboina, R.R.Y.; Cheng, Y.; Zhou, C.; et al. Novel Schiff base-bridged multi-component sulfonamide imidazole hybrids as potentially highly selective DNA-targeting membrane active repressors against methicillin-resistant Staphylococcus aureus. Bio. Org. 2021, 107, 104575. [Google Scholar] [CrossRef]
- Basarab, G.S.; Hill, P.; Eyermann, C.J.; Gowravaram, M.; Käck, H.; Osimoni, E. Design of inhibitors of Helicobacter pylori glutamate racemase as selective antibacterial agents: Incorporation of imidazoles onto a core pyrazolopyrimidinedione scaffold to improve bioavailabilty. Bioorg. Med. Chem. Lett. 2012, 17, 5600–5607. [Google Scholar] [CrossRef]
- Gobis, K.; Foks, H.; Serocki, M.; Augustynowicz-Kope, E.; Napiórkowska, A. Synthesis and evaluation of in vitro antimycobacterial activity of novel 1H-benzo[d]imidazole derivatives and analogues. Eur. J. Med. Chem. 2015, 89, 13–20. [Google Scholar] [CrossRef]
- Daraji, D.G.; Rajani, D.P.; Rajani, D.S.; Pithawala, E.A.; Jayanthi, S.; Patel, H.D. Structure based design, synthesis, and biological evaluation of imidazole derivatives targeting dihydropteroate synthase enzyme. Bioorg. Med. Chem. Lett. 2021, 36, 127819. [Google Scholar] [CrossRef] [PubMed]
- Molina, P.; Tárraga, A.; Otón, F. Imidazole derivatives: A comprehensive survey of their recognition properties. Org. Biomol. Chem. 2012, 10, 1711–1724. [Google Scholar] [CrossRef] [PubMed]
- Bulut, O.; Oktem, H.A.; Yilmaz, M.D. A highly substituted and fluorescent aromatic-fused imidazole derivative that shows enhanced antibacterial activity against Methicillin-resistant Staphylococcus Aureus (MRSA). J. Hazard. Mater. 2020, 399, 122902. [Google Scholar] [CrossRef]
- Zheng, X.; Ma, Z.; Zhang, D. Synthesis of imidazole-based medicinal molecules utilizing the van Leusen imidazole synthesis. Pharmaceuticals 2020, 13, 37. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Song, G.; Sun, M.; Wang, J.; Wang, Y. Prevalence and therapies of antibiotic-resistance in Staphylococcus Aureus. In Front. Cell. Infect. Microbiol. 2020, 10, 107. [Google Scholar] [CrossRef]
- Nagarajan, N.; Vanitha, G.; Ananth, D.A.; Rameshkumar, A.; Sivasudha, T.; Renganathan, R. Bioimaging, antibacterial and antifungal properties of imidazole-pyridine fluorophores: Synthesis, characterization and solvatochromism. J. Photo. Biol. 2013, 127, 212–222. [Google Scholar] [CrossRef]
- Valls, A.; Andreu, J.J.; Falomir, E.; Luis, S.V.; Atrián-Blasco, E.; Mitchell, S.G.; Altava, B. Imidazole and Imidazolium Antibacterial Drugs Derived from Amino Acids. Pharmaceuticals 2020, 13, 482. [Google Scholar] [CrossRef]
- Zhang, L.; Peng, X.-M.; Damu, G.L.V.; Geng, R.-X.; Zhou, C.-H. Comprehensive review in current developments of imidazole-based medicinal chemistry. Med. Res. Rev. 2014, 34, 340–437. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, T.Q.; Chen, Y.; Chen, X.D.; Chang, H.K.; Zhang, Y.M.; Hua, S.C. Microwave-assisted solvent-free synthesis and luminescence properties of 2-substituted-4,5-di(2-furyl)-1H-imidazoles. Chem. Pap. 2014, 69, 325–338. [Google Scholar] [CrossRef]
- Kidwai, M.; Mothsra, P.; Bansal, V.; Somvanshi, R.K.; Ethayathulla, A.S.; Dey, S.; Singh, T.P. One-pot synthesis of highly substituted imidazoles using molecular iodine: A versatile catalyst. J. Mol. Catal. A Chem. 2007, 265, 177–182. [Google Scholar] [CrossRef]
- Morris, J.V.; Mahaney, M.A.; Huber, J.R. Fluorescence quantum yield determinations. 9,10-Diphenylanthracene as a reference standard in different solvents. J. Phys. Chem. 2002, 80, 969–974. [Google Scholar] [CrossRef]
- Yuan, L.; Lin, W.; Zheng, K.; He, L.; Huang, W. Far-red to near infrared analyte-responsive fluorescent probes based on organic fluorophore platforms for fluorescence imaging. Chem. Soc. Rev. 2013, 42, 622–661. [Google Scholar] [CrossRef]
- Ramos, N.; Costa, S.P.G.; Oliveira, R.; Raposo, M.M.M. Synthesis and preliminary antibacterial evaluation of a 2,4,5-tri(hetero)arylimidazole derivative. Chem. Proc. 2021, 3, 41. [Google Scholar] [CrossRef]

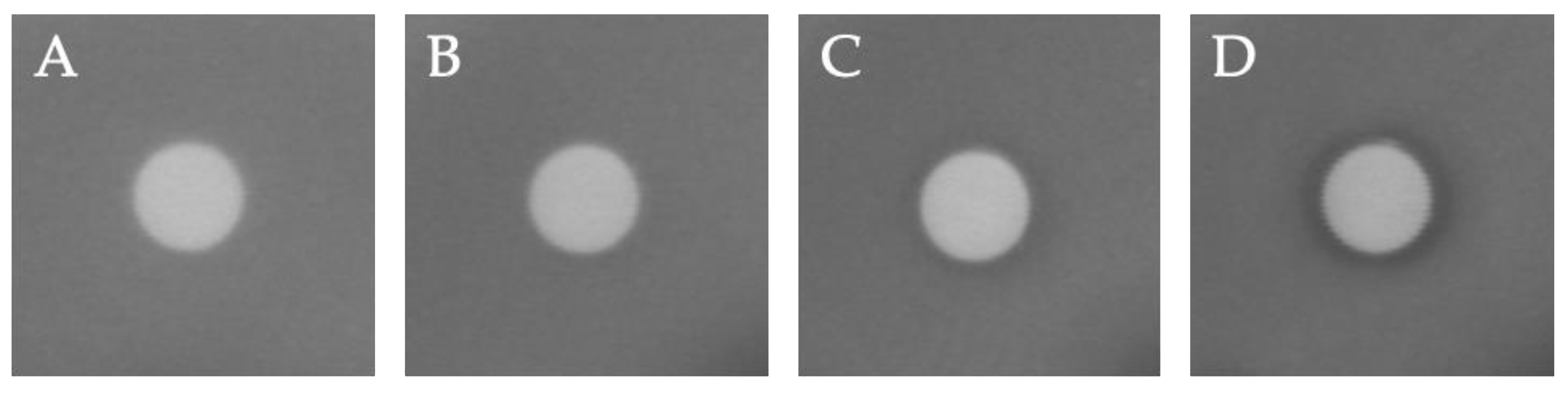
| Compound | 3 μg/disk | 5 μg/disk | 15 μg/disk | 30 μg/disk |
|---|---|---|---|---|
| 3 | - | ND | ND | (8 ± 1) |
| Ampicillin | (23.0 ± 0.1) | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos, N.L.P.; Oliveira, R.; Costa, S.P.G.; Raposo, M.M.M. Synthesis, Characterization and Preliminary Antibacterial Evaluation against Staphylococcus aureus of a New 2,4,5-Tri(hetero)arylimidazole Derivative Based on Azaindole Heterocycle. Chem. Proc. 2022, 8, 104. https://doi.org/10.3390/ecsoc-25-11781
Ramos NLP, Oliveira R, Costa SPG, Raposo MMM. Synthesis, Characterization and Preliminary Antibacterial Evaluation against Staphylococcus aureus of a New 2,4,5-Tri(hetero)arylimidazole Derivative Based on Azaindole Heterocycle. Chemistry Proceedings. 2022; 8(1):104. https://doi.org/10.3390/ecsoc-25-11781
Chicago/Turabian StyleRamos, Nuna L. P., Rui Oliveira, Susana P. G. Costa, and Maria Manuela M. Raposo. 2022. "Synthesis, Characterization and Preliminary Antibacterial Evaluation against Staphylococcus aureus of a New 2,4,5-Tri(hetero)arylimidazole Derivative Based on Azaindole Heterocycle" Chemistry Proceedings 8, no. 1: 104. https://doi.org/10.3390/ecsoc-25-11781
APA StyleRamos, N. L. P., Oliveira, R., Costa, S. P. G., & Raposo, M. M. M. (2022). Synthesis, Characterization and Preliminary Antibacterial Evaluation against Staphylococcus aureus of a New 2,4,5-Tri(hetero)arylimidazole Derivative Based on Azaindole Heterocycle. Chemistry Proceedings, 8(1), 104. https://doi.org/10.3390/ecsoc-25-11781







