The Molecular Species Identified by GS-MS in Sol-Gel Process. Operational Mass Spectrum Libraries †
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Badescu, V. Reactivitatea Alcoxizilor Studiata Prin Reactii Ionice in Faza Gazoasa. Ph.D. Thesis, Roumanian Academy, Bucharest, Romania, 1998. [Google Scholar]
- Badescu, V.; Radu, M.; Zaharescu, M.; Vasilescu, A. Hydrolysis-condentation of tetraethoxysilane in nonparental solvents studied by GC-MS. J. Sol-Gel Sci. Technol. 1994, 2, 43–49. [Google Scholar] [CrossRef]
- Zaharescu, M.; Vasilescu, A.; Badescu, V.; Radu, M. Hydrolysis-polycondensation in binary Phosphorous alcoxides-TEOS system studied by GC-MS. J. Sol-Gel Sci. Technol. 1997, 8, 59–63. [Google Scholar] [CrossRef]
- Badescu, V.; Zaharescu, M.; Vasilescu, A.; Radu, M. The Influence of Solvent on Hydrolysis and Condensation of Tetraethoxysilane Studied by GC-MS. Rev. Roum. Chim. 1996, 41, 733–740. [Google Scholar]
- Zaharescu, M.; Badescu, V.; Vasilescu, A.; Radu, M. Influence of the catalyst on the Hydrolysis-Condensation of Tetraethoxysilane Studied by GC-MS. Rev. Roum. Chim. 1997, 42, 633–639. [Google Scholar]
- Badescu, V. Contributions to the interpretation of mass spectrum of tetraethoxysilane (TEOS). Part I: The molecular ion. Primary events. Rev. Roum. Chim. 2014, 59, 875–882. [Google Scholar]
- Badescu, V. Contributions to the interpretation of mass spectrum of tetraethoxysilane (TEOS). Part II: Charge induced reactions. Eliminations of neutral fragments. Rev. Roum. Chim. 2015, 60, 1107–1115. [Google Scholar]
- Badescu, V. Contributions to the interpretation of mass spectrum of hexaethoxydisiloxane. Linked scans and M+1, M+2 isotopic effects. Rev. Chim. 2019, 70, 2934–2939. [Google Scholar] [CrossRef]
- Badescu, V. Contributions to the interpretation of mass spectrum of tetraethoxysiloxane. Part III Accurate mass and M+1, M+2 isotopic effects. Rev. Chim. 2020, 71, 209–217. [Google Scholar] [CrossRef]
- Badescu, V. Contributions to the interpretation of mass spectra of the TEOS methoxy- transesters. Linked scans, accurate mass measurements and M+1, M+2 isotopic effects. Rev. Chim. 2020, 71, 39–54. [Google Scholar] [CrossRef]
- PerkinElmer. TurboMass Software User’s Guide; PerkinElmer, Inc.: Waltham, MA, USA, 2007; pp. 525–532. [Google Scholar]
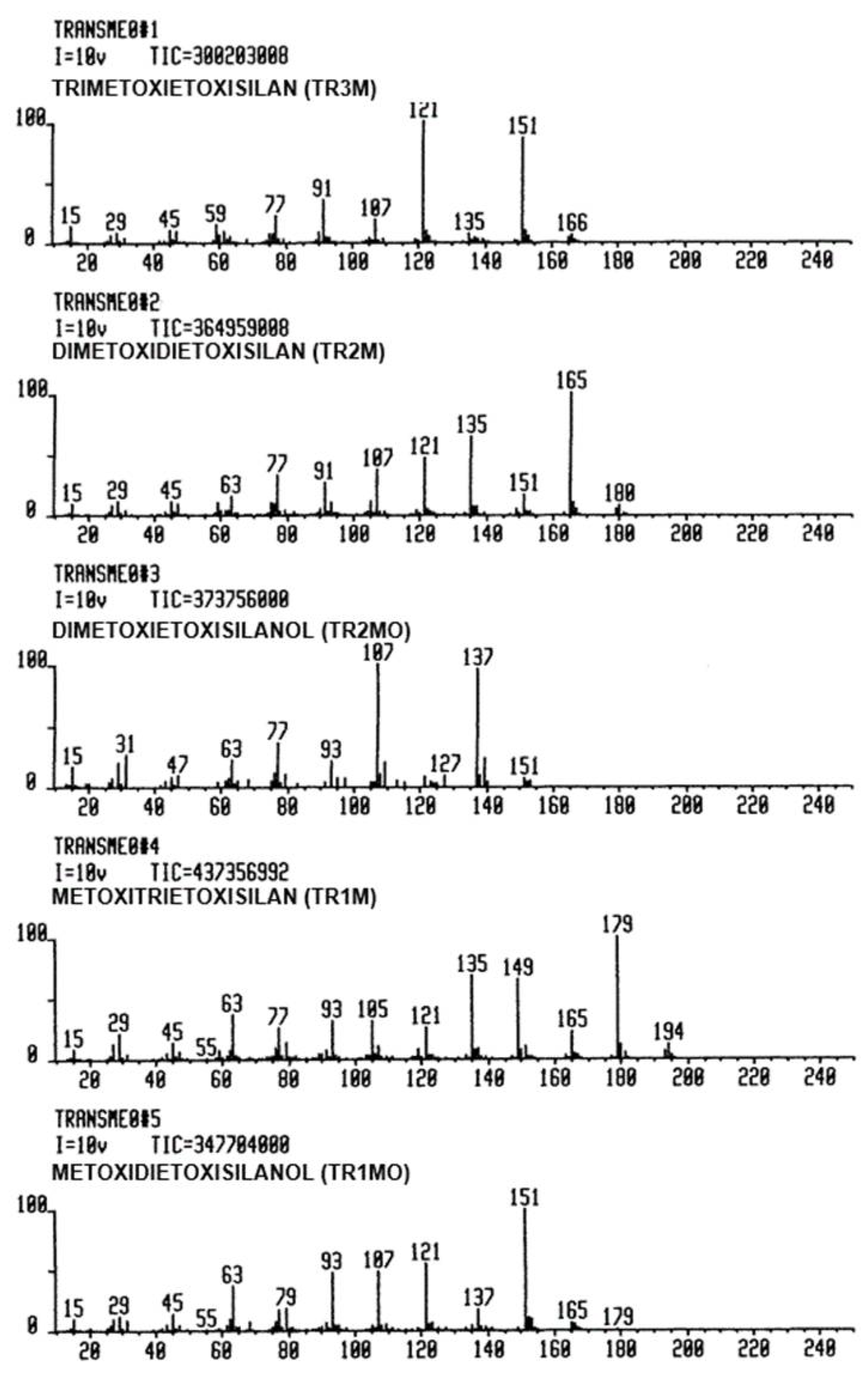
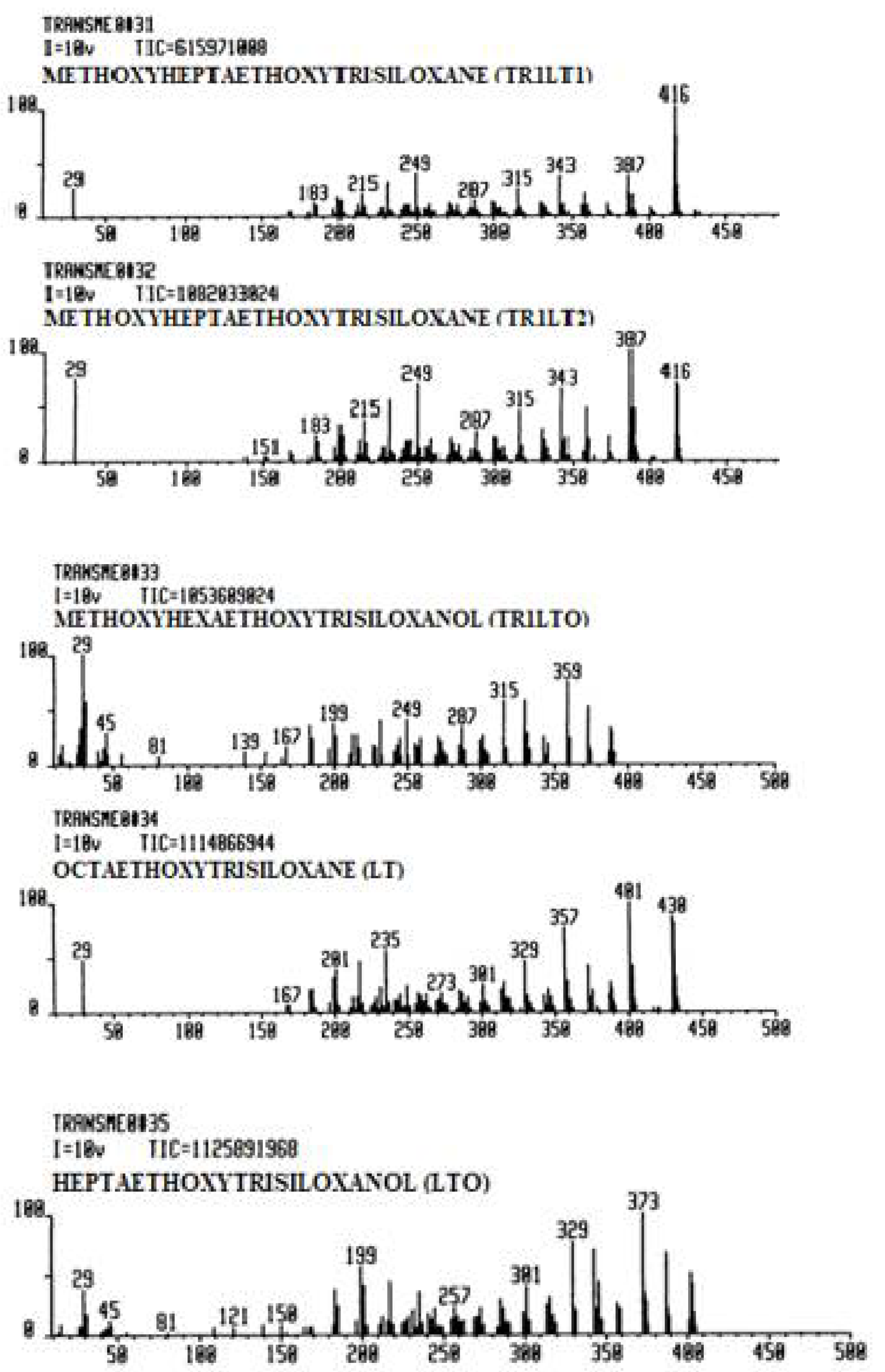
| Reaction Systems | Mass Spectrum Library Name Code | Number of Entries | Observations |
|---|---|---|---|
| TEOS: H2O: EtOH | ICECHIM0 to ICECHIM9 | 10–33 | start mixture to 9 days |
| TEOS: H2O: MeOH | TRANSME0 | 35 | methoxy transesters |
| TEOS: H2O: PrOH | TRANSPR1 | 19 | propoxy transesters |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badescu, V. The Molecular Species Identified by GS-MS in Sol-Gel Process. Operational Mass Spectrum Libraries. Chem. Proc. 2022, 7, 73. https://doi.org/10.3390/chemproc2022007073
Badescu V. The Molecular Species Identified by GS-MS in Sol-Gel Process. Operational Mass Spectrum Libraries. Chemistry Proceedings. 2022; 7(1):73. https://doi.org/10.3390/chemproc2022007073
Chicago/Turabian StyleBadescu, Virgil. 2022. "The Molecular Species Identified by GS-MS in Sol-Gel Process. Operational Mass Spectrum Libraries" Chemistry Proceedings 7, no. 1: 73. https://doi.org/10.3390/chemproc2022007073
APA StyleBadescu, V. (2022). The Molecular Species Identified by GS-MS in Sol-Gel Process. Operational Mass Spectrum Libraries. Chemistry Proceedings, 7(1), 73. https://doi.org/10.3390/chemproc2022007073





