Fungi are known for their capacity to produce two main categories of enzymes, cellulolytic and lignolytic, both valuable for biodegradation of lignocellulosic biomass. Ganoderma lucidum represents one of the widely grown basidiomycete white fungi for the production of lignolytic enzymes. Co-cultures of macrofungi with different microorganisms were previously shown to boost the production of bioactive components and expression of functional enzymes [1]. Our aim was to co-cultivate G. lucidum with several bacterial strains in order to identify optimal co-cultures that increase the production of lignolytic enzymes [2].
G. lucidum was tested in interactions with 9 strains of bacteria. The growth medium used was potato dextrose agar (PDA) for the synergistic–antagonistic test since both fungi and the bacterial species studied grew well on the medium and potato dextrose broth (PDB) for the enzymatic study [3]. All enzymatic activities were determined by UV-Vis spectroscopy. Laccase (Lac) activity was determined measuring the absorbance of ABTS at 420 nm (ε = 36,000 M−1cm−1), Ligninperoxidase (LiP) the oxidation of veratryl alcohol at 310 nm (ε = 9300 M−1cm−1), Manganese peroxidase (MnP) the absorbance of 2,6-dimethoxyphenol at 469 nm (ε = 53,200 M−1cm−1) and Veratryl alcohol oxidase (VAO) the absorbance of veratryl alcohol at 310 nm (ε = 9300 M−1cm−1) [4].
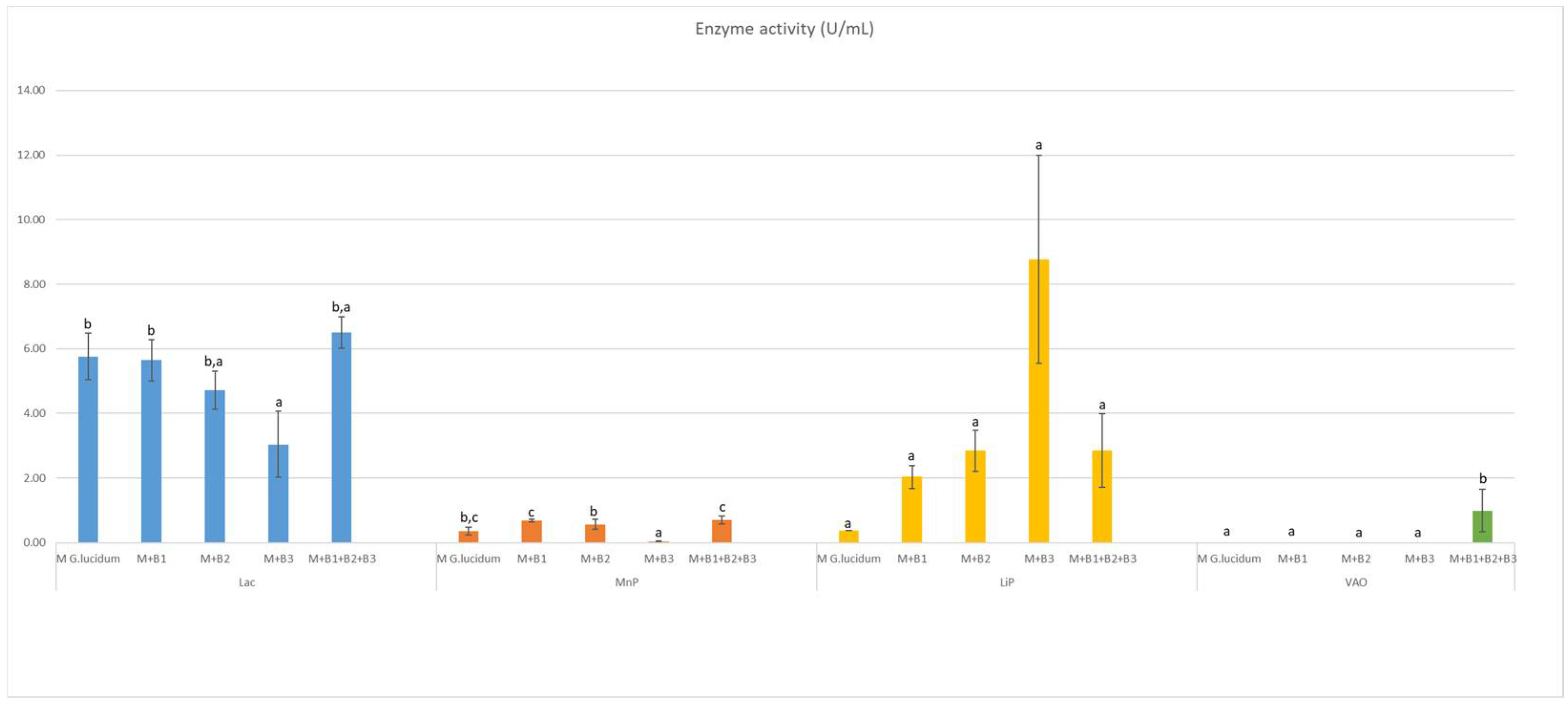
In the case of Lac, there was no significant improvement in the enzymatic activity provided by co-cultivation of G. lucidum with bacteria. For MnP and VAO, there was a slight increase in the activity for the experimental variants containing G. lucidum and bacterial strains. LiP activity improved significantly due to co-cultivation. The bacteria cultures did not exhibit enzymatic activity.
We co-cultivated G. lucidum with several bacterial strains, achieving an improvement in the activity of certain lignolytic enzymes—Figure 1.
Figure 1.
Enzyme activity of Lac, MnP, LiP and VAO in (U/mL), where a, b, and c are homogenoues subsets (from one way ANOVA analysis).
Author Contributions
Conceptualization, F.O. and D.P.; methodology, D.-G.P.; software, D.P.; validation, D.-G.P., D.C.-A. and F.O.; formal analysis, D.P.; investigation, D.P.; resources, F.O.; data curation, D.C-A.; writing—original draft preparation, D.P.; writing—review and editing, D.C.-A. and F.O.; visualization, D.P.; supervision, F.O.; project administration, F.O.; funding acquisition, F.O. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by project POC-A1-A1.2.3-G-2015-P_40_352-SECVENT, My_SMIS 105684, “Sequential processes of closing the side streams from bioeconomy and innovative (bio)products resulting from it, subsidiary project 382/2021 “Cellulose bleaching by using enzymes produced by consensus microbial consortia and environmentally friendly oxidizing agents”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yu, G.; Sun, Y.; Han, H.; Yan, X.; Wang, Y.; Ge, X.; Qiao, B.; Tan, L. Coculture, an efficient biotechnology for mining the biosynthesis potential of macrofungi via interspecies interactions. Front. Microbiol. 2021, 12, 1–14. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, T.M.; Merritt, C.S.; Reddy, C.A. Lignin-modifying enzymes of the white rot basidiomycete Ganoderma lucidum. Appl. Environ. Microbiol. 1999, 65, 5307–5313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jo, W.-S.; Cho, Y.-J.; Cho, D.-H.; Park, S.-D.; Yoo, Y.-B.; Seok, S.-J. Culture conditions for the mycelial growth of Ganoderma applanatum. Mycobiology 2009, 37, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, A.; Chandra, R. Ligninolytic enzymes and its mechanisms for degradation of lignocellulosic waste in environment. Heliyon 2020, 6, e03170. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).