Synthesis of Silver Nanoparticles Embedded in Micro-Hydrogel Particles by Electron Beam Irradiation †
Abstract
:1. Introduction
2. Materials and Methods
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lungulescu, E.M. Gamma Radiation Synthesis of Colloidal Silver Nanoparticles. Rev. Chim. 2019, 70, 2826–2850. [Google Scholar] [CrossRef]
- Popa, M.; Măruţescu, L.; Ion, I.; Kamerzan, C.; Bleotu, C.; Oprea, E.; Chifiriuc, M.C.; Lazăr, V. Chapter14—Antimicrobial and cytotoxic activity of graphene based perioceuticals. In Fullerens, Graphenes and Nanotubes, 1st ed.; Grumezescu, A.M., Deans, M., Eds.; Elsevier: Bucharest, Romania, 2018; pp. 585–599. ISBN 978-0-12-813691-1. [Google Scholar] [CrossRef]
- Fierascu, R.C.; Fierascu, I.; Lungulescu, E.M.; Nicula, N.; Somoghi, R.; Diţu, L.M.; Ungureanu, C.; Sutan, A.N.; Draghiceanu, O.A.; Paunescu, A.; et al. Phytosynthesis and radiation-assisted methods for obtaining metal nanoparticles. J. Mater. Sci. 2020, 55, 1915–1932. [Google Scholar] [CrossRef]
- Mehrali, M.; Bagherifard, S.; Akbari, M.; Thakur, A.; Mirani, B.; Mehrali, M.; Hasany, M.; Orive, G.; Das, P.; Emneus, J.; et al. Blending Electronics with the Human Body: A Pathway toward a Cybernetic Future. Adv. Sci. 2018, 5, 1700931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, J.-O.; Park, J.-S.; Kim, Y.-A.; Yang, S.-J.; Jeong, S.-I.; Lee, J.-Y.; Lim, Y.-M. Gamma Ray-Induced Polymerization and Cross-Linking for Optimization of PPy/PVP Hydrogel as Biomaterial. Polymers 2020, 12, 111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Telipan, G.; Pislaru-Danescu, L.; Ion, I.; Marinescu, V. Ag/polypyrrole composites for polarizable dry microsensor used in bioimpedance measurement. In Proceedings of the 2020 International Semiconductor Conference (CAS), Sinaia, Romania, 7–9 October 2020; pp. 23–26. [Google Scholar] [CrossRef]
- Hummer, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Mogaldea, G.; Ion, I.; Sandu, O.; Stamatin, I.; Dumitru, B.A.; Mogaldea, M. Structural investigation of the graphite oxide and thermal reduced graphite oxide with terahertz spectroscopy. Optoelectron. Adv. Mat. 2011, 5, 973–976. [Google Scholar]
- Manaila, E.; Craciun, G.; Ighigeanu, D.; Campeanu, C.; Barna, C.; Fugaru, V. Hydrogels synthesized by electron beam irradiation for heavy metal adsorption. Materials 2017, 10, 540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bahram, M.; Mohseni, N.; Moghtader, M. Chapter—An Introduction to Hydrogels and Some Recent. In Emerging Concepts in Analysis and Applications of Hydrogels, 1st ed.; Majee, S.B., Ed.; IntechOpen: London, UK, 2016. [Google Scholar] [CrossRef] [Green Version]
- Zheng, X.; Peng, Y.; Cui, X.; Zheng, W. Modulation of the shape and localized surface plasmon resonance of silver nanoparticles via halide ion etching and photochemical regrowth. Mater. Lett. 2016, 173, 88–90. [Google Scholar] [CrossRef]
- Paramelle, D.; Sadovoy, A.; Gorelik, S.; Free, P.; Hobleya, J.; Fernig, D.G. A rapid method to estimate the concentration of citrate capped silver nanoparticles from UV-visible light spectra. Analyst 2014, 139, 4855–4861. [Google Scholar] [CrossRef] [PubMed]
- Peppas, N.A.; Merrill, E.W. Differential scanning calorimetry of crystallized PVA hydrogels. J. Appl. Polym. Sci. 1976, 20, 1457–1465. [Google Scholar] [CrossRef]
- Fathi, E.; Atyabi, N.; Imani, M.; Alinejad, Z. Physically crosslinked polyvinyl alcohol–dextran blend xerogels: Morphology and thermal behavior. Carbohydr. Polym. 2011, 84, 145–152. [Google Scholar] [CrossRef]
- Gupta, S.; Pramanik, A.K.; Kailath, A.; Mishra, T.; Guha, A.; Nayar, S.; Sinha, A. Composition dependent structural modulations in transparent poly(vinyl alcohol) hydrogels. Coll. Surf. B Biointerfaces 2009, 74, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Montoya-Villegas, K.A.; Ramírez-Jiménez, A.; Licea-Claverie, Á.; Pérez-Sicairos, S.; Bucio, E.; Bernáldez-Sarabia, J.; Licea-Navarro, A.F. Surface Modification of Polyester-Fabric with Hydrogels and Silver Nanoparticles: Photochemical Versus Gamma Irradiation Methods. Materials 2019, 12, 3284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bavaresco, V.P.; Machado, L.D.B.; Pino, E.S.; Zavaglia, C.A.C.; Reis, M.C. Effect of the irradiation process on the properties of PVA hydrogels to be used as biomaterial. In Proceedings of the 2007 International Nuclear Atlantic Conference—INAC 2007, São Paulo, Brazil, 30 September–5 October 2007. [Google Scholar]
- Sheela, T.; Bhajantri, R.F.; Ravindrachary, V.; Rathod, S.G.; Pujari, P.K.; Poojary, B.; Somashekar, R. Effect of UV irradiation on optical, mechanical and microstructural properties of PVA/NaAlg blends Radiat. Phys. Chem. 2014, 103, 45–52. [Google Scholar]
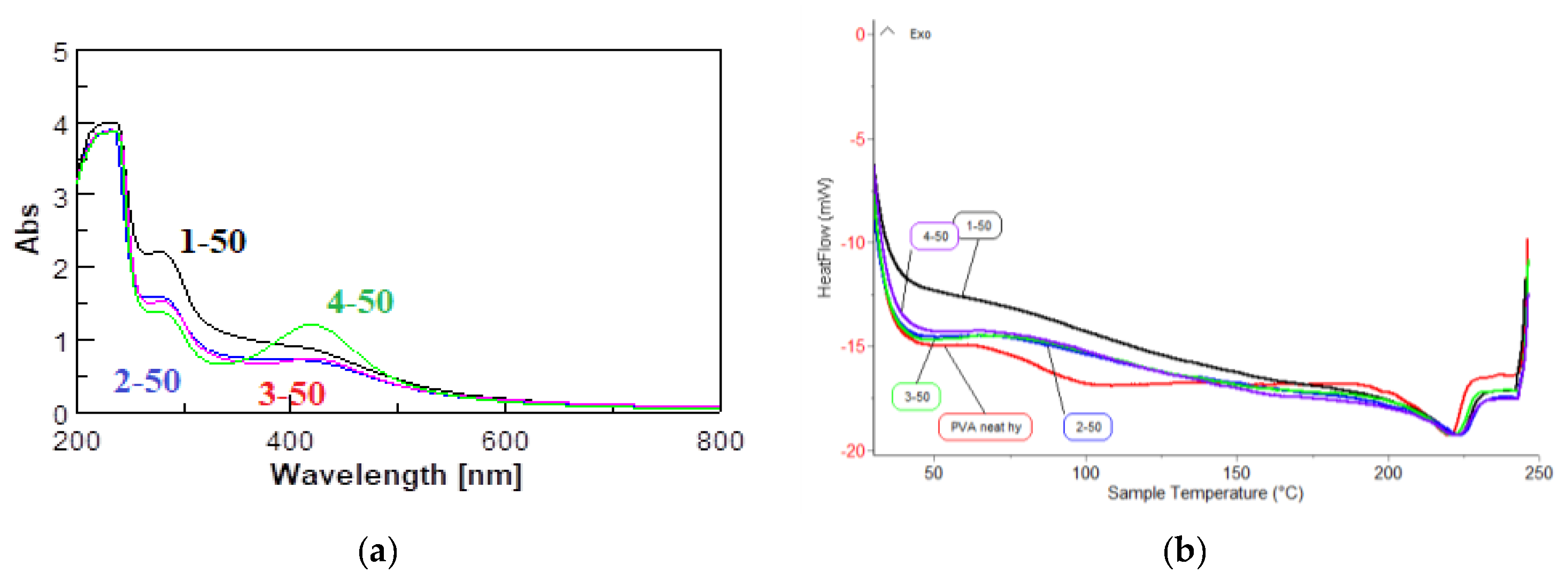

| HMH Codification | Batch 10 kGy | Batch 25 kGy | Batch 50 kGy | |||
|---|---|---|---|---|---|---|
| λmax [nm] | Abs [a.u.] | λmax [nm] | Abs [a.u.] | λmax [nm] | Abs [a.u.] | |
| 1.HMH PVA-Py-Fe-Ag | - | - | - | - | 380 | 0.73 |
| 2.HMH PVA Py-Fe-Ag-GO | - | - | - | - | 397.5 | 0.68 |
| 3.HMH PVA Py-Ag | 417 | 1.24 | 418.5 | 0.84 | 419 | 0.7 |
| 4.HMHPVA Py-Ag-GO | 416 | 1.59 | 417.5 | 1.50 | 419.5 | 1.21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ion, I.; Stancu, E.; Mitu, C.M.; Marinescu, V.; Lungulescu, E.M.; Nicula, N.O. Synthesis of Silver Nanoparticles Embedded in Micro-Hydrogel Particles by Electron Beam Irradiation. Chem. Proc. 2022, 7, 22. https://doi.org/10.3390/chemproc2022007022
Ion I, Stancu E, Mitu CM, Marinescu V, Lungulescu EM, Nicula NO. Synthesis of Silver Nanoparticles Embedded in Micro-Hydrogel Particles by Electron Beam Irradiation. Chemistry Proceedings. 2022; 7(1):22. https://doi.org/10.3390/chemproc2022007022
Chicago/Turabian StyleIon, Ioana, Elena Stancu, Ciprian Mihai Mitu, Virgil Marinescu, Eduard Marius Lungulescu, and Nicoleta Oana Nicula. 2022. "Synthesis of Silver Nanoparticles Embedded in Micro-Hydrogel Particles by Electron Beam Irradiation" Chemistry Proceedings 7, no. 1: 22. https://doi.org/10.3390/chemproc2022007022
APA StyleIon, I., Stancu, E., Mitu, C. M., Marinescu, V., Lungulescu, E. M., & Nicula, N. O. (2022). Synthesis of Silver Nanoparticles Embedded in Micro-Hydrogel Particles by Electron Beam Irradiation. Chemistry Proceedings, 7(1), 22. https://doi.org/10.3390/chemproc2022007022







