Abstract
NADES represent a new generation of biocompatible solvents that are formed by eutectic mixtures of two or more hydrogen bond donor and hydrogen bond acceptor compounds of natural origin and that have a lower melting point compared to pure components. The ease of preparation, sustainability, low cost, and low toxicity of NADES have allowed these solvents to be investigated in biocatalysis. In this communication, we describe the stability and the enzymatic activity of two oxidoreductases, HLADH and TsER, in buffer solution and in choline-based NADES/buffer mixtures. In particular, we report on the enantioselective lactonization of 3-methyl-1,5-pentanediol into 4-methyl-δ-valerolactone and the bioreduction of ketoisophorone into levodione.
1. Introduction
Natural Deep Eutectic Solvents (NADES) are a group of molecular solvents that are entirely composed of plant primary metabolites such as sugars, alcohols, amino acids, organic acids, and choline derivatives [1]. They are composed of salts such as choline chloride (C), which act as hydrogen bond acceptors (HBAs), and hydrogen bond donors (HBDs), which are capable of associating through hydrogen bonds and van der Waals interactions to form eutectic mixtures with melting points that are much lower than those of single components. Due to properties such as non-volatility, non-flammability, low toxicity, and biodegradability, NADES are considered to be green solvents and have started to be assessed as tools for biocatalysis, either for use as solvents or as separative agents, with the aim of eliminating the multiple steps that are involved in complex chemical syntheses [2].
The use of oxidoreductases in industrial organic synthesis has been gaining momentum in the last decade. In this field, horse liver alcohol dehydrogenase (HLADH) is one of the most popular biocatalysts for the stereoselective oxidation of alcohols due to its commercial availability and stereospecificity. This zinc-dependent enzyme demonstrates oxidant activity using oxidized nicotinamide cofactors (NAD(P)+) as electron acceptors [3]. HLADH is the catalyst of choice for the direct oxidative lactonization of diols [4]; in particular, 3-methyl-1,5-pentanediol is the substrate for the synthesis of (S)-3-methyl-δ-valerolactone, an interesting building block that is present in some complex natural products [5]. Enoate reductases (ERs) are members of the “old yellow enzyme” family, a class of flavin-dependent enzymes that catalyze the reduction of C=C bonds that have been conjugated with electron-withdrawing groups with absolute stereospecificity at the expense of a nicotinamide cofactor. A homologous enzyme platform was developed, and several industrially relevant molecules were obtained in an enantiomerically active form [6]. α,β-Enones are usually well-accepted substrates for ERs; in particular, after asymmetric bioreduction, the 4-ketoisophorone furnishes (R)-levodione, an important industrial intermediate for carotenoide synthesis [7].
In this communication, we report studies on HLADH and ER from Thermus scotoductus (TsER), evaluating the enzyme activity and enantioselectivity during the lactonization of 3-methyl-1,5-pentanediol and ketoisophorone bioreduction, respectively, with xylitol (Xo)/choline chloride (C)/distilled water (H) as NADES. Our aim is to associate the biocatalysis and the use of low-toxicity solvents (NADES) as two important green approaches for the preparation of chiral building blocks.
2. Methods
Commercially available reagents were used without further purification unless stated otherwise. All solutions were mixed and thermostated using a Thermomixer comfort (Eppendorf AG, Hamburg, Germany). GC analyses were performed on a Shimadzu GC-2010 plus, Chiraldex GTA columns (30 m × 0.25 mm × 0.12 µm) for HLADH and MEGA-DEX DET beta columns (25 m × 0.25 mm × 0.25 μm) for TsER, and an FID detector.
XoCH preparation: C was recrystallized from absolute ethanol, filtered, and dried under vacuum. Xo was dried under vacuum prior to use. The components were mixed with the calculated amount of H (XoCH 1:2:3 ratio), heated to 50 °C, and stirred until a clear and viscous liquid was formed (after about 60–90 min). The liquid was filtered under a vacuum, used without further purification, and stored at 4 °C.
3. Results and Discussion
3.1. HLADH Catalyzes the Lactonization of 3-Methyl-1,5-Pentanediol
The stability of HLADH was assessed in XoCH by means of UV/Vis spectroscopy to measure the formation of NADH. XoCH caused a remarkable decrease in enzyme stability when mixed at 25% and 75% in amino-2-(hydroxymethyl)propane-1,3-diol (TRIS)-HCl. The stability of the enzyme was only ameliorated in XoCH/TRIS-HCl 50% with respect to pure buffer. Subsequently, the synthesis of (S)-3-methyl-δ-valerolactone from 3-methyl-1,5-pentanediol catalyzed by HLADH [4] was initially performed in a watery solution of 2-amino-2-(hydroxymethyl)propane-1,3-diol (TRIS)-HCl, and the NADES (XoCH/TRIS-HCl mixtures) were introduced at various concentrations until a pure NADES concentration (100%) was reached. A reaction temperature of 30 °C was chosen to optimize HLADH activity and to decrease NADES viscosity. The reaction was followed with chiral GC for 24 h, highlighting the disappearance of 3-methyl-1,5-pentanediol and the formation of (S)-3-methyl-δ-valerolactone (Scheme 1).
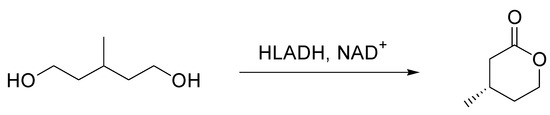
Scheme 1.
Reaction conditions: (substrate) = (cofactor) = 5 mMn (HLADH) = 0.2 mg/mL, TRIS-HCl or mixtures XoCH/TRIS-HCl, 30 °C, 24 h.
The formation of the lactone in its enantiomerically pure form as a function of the solvent composition is shown in Figure 1.
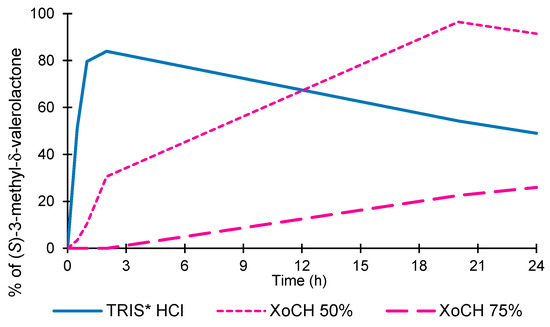
Figure 1.
Synthesis of 3-methyl-δ-valerolactone via HLADH in TRIS-HCl and XoCH/TRIS-HCl (25%, 50%, and 75% w/w).
Full substrate conversion was obtained in 100% TRIS-HCl after 2 h. Subsequently, a slight decrease in the concentration of the final product was observed. With 50% XoCH/buffer, the formation of the product was more gradual, reaching a maximum concentration value after 20 h. As the NADES percentage increased (75%), the concentration of the final product was greatly reduced. The curve for 100% XoCH is missing, as no reaction occurred. The enantioselectivity evaluation also showed that the reaction was stereospecific; in particular, when the NADES concentration increased from 0% to 50%, there was a substantial increase in the stereoselectivity (99%), which was maintained until a NADES concentration of 75% was achieved.
3.2. TsER Catalyzes the Reduction of Ketoisophorone
Similar to the previous enzyme, the TsER stability was assessed in XoCH at different concentrations. The results were satisfactory, and no substantial changes were detected in the enzyme. The bioreduction of ketoisophorone catalyzed by TsER resulted in (R)-levodione as the prevailing product when the reaction was conducted in 3-(N-morpholino)propanesulfonic acid (MOPS) buffer with CaCl2 at pH 7.0 [8] and in MOPS with increasing concentrations of XoCH until pure XoCH was reached at 40 °C. The reaction was followed by chiral GC for 24 h, highlighting the disappearance of the ketoisophorone and the formation of levodione (Scheme 2).
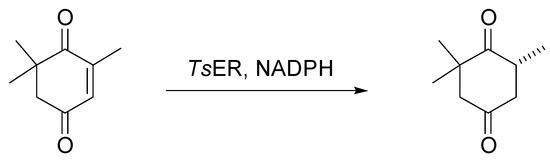
Scheme 2.
Reaction conditions: (substrate) = 10 mM, (NADH) = 15 mM, (TsER) = 1 mg/mL, MOPS, mixtures XoCH/MOPS or pure XoCH, 40 °C, 24 h.
The formation of (R)-levodione as a function of the solvent composition is reported in Figure 2.
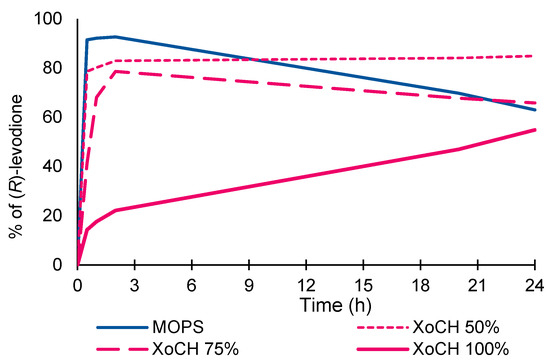
Figure 2.
Synthesis of (R)-levodione via TsER in MOPS, XoCH in MOPS (50%, 75% w/w), and pure XoCH.
The full conversion of ketoisophorone into levodione was obtained with pure MOPS. With 50% and 75% concentrations of XoCH/buffer, the formation of the product was complete in 2 h, and there was a decrease in the concentration of final product in the case of the 75% NADES concentration after 20 h. With pure XoCH, product formation decreased, but a progressive increase in the concentration was observed over the 24 h reaction time. The enantioselectivity evaluation of the reaction also showed that the R/S ratio of levodione increased slightly with the NADES concentration, reaching high XoCH values above 75%.
4. Conclusions
In summary, we conducted two enantioselective reactions: the lactonization of 3-methyl-1,5-pentanediol and the reduction of ketoisophorone catalyzed by the redox enzymes HLADH and TsER, respectively, using various concentrations of XoCH as the solvent (mixed with buffer). In both cases, we noticed that NADES favor the activity of the two enzymes as well as stereoselectivity, but only at defined concentrations. Overall, these results suggest that NADES possess good biocompatibility with the studied enzymes and could contribute to creating environmentally friendly processes.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The author declares no conflict of interest.
References
- Paiva, A.; Craveiro, R.; Aroso, I.; Martins, M.; Reis, R.L.; Duarte, A.R.C. Natural Deep Eutectic Solvents—Solvents for the 21st century. ACS Sustain. Chem. Eng. 2014, 2, 1063–1071. [Google Scholar] [CrossRef]
- Xia, N.; Xiong, L.; Bi, S.; Qian, F.; Wang, P. Development of biocompatible DES/NADES as co-solvents for efficient biosynthesis of chiral alcohols. Bioprocess Biosyst. Eng. 2020, 43, 1987–1997. [Google Scholar] [CrossRef] [PubMed]
- Plapp, B.V.; Savarimuthu, B.R.; Ferraro, D.J.; Rubach, J.K.; Brown, E.N.; Ramaswamy, S. Horse Liver Alcohol Dehydrogenase: Zinc coordination and catalysis. Biochemistry 2017, 56, 3632–3646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kara, S.; Spickermann, D.; Schrittwieser, J.H.; Weckbecker, A.; Leggewie, C.; Arends, I.W.C.E.; Hollmann, F. Access to lactone building blocks via Horse Liver Alcohol Dehydrogenase-catalyzed oxidative lactonization. ACS Catal. 2013, 3, 2436–2439. [Google Scholar] [CrossRef]
- Fischer, T.; Pietruszka, J. Alcohol dehydrogenase-catalyzed synthesis of enantiomerically pure delta-lactones as versatile intermediates for natural product synthesis. Adv. Synth. Catal. 2012, 354, 2521–2530. [Google Scholar] [CrossRef]
- Scholtissek, A.; Tischler, D.; Westphal, A.H.; van Berkel, W.J.H.; Paul, C.E. Old yellow enzyme-catalysed asymmetric hydrogenation: Linking family roots with improved catalysis. Catalysts 2017, 7, 130. [Google Scholar] [CrossRef]
- Winkler, C.K.; Tasnádi, G.; Clay, D.; Hall, M.; Faber, K. Asymmetric bioreduction of activated alkenes to industrially relevant optically active compounds. J. Biotechnol. 2012, 162, 381–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paul, C.E.; Gargiulo, S.; Opperman, D.J.; Lavandera, I.; Gotor-Fernandez, V.; Gotor, V.; Taglieber, A.; Arends, I.W.C.E.; Hollmann, F. Mimicking nature: Synthetic nicotinamide cofactors for C=C bioreduction using Enoate Reductases. Org. Lett. 2013, 15, 180–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).