1. Introduction
Metals have been deposited on several supports (e.g., carbon materials, metallic oxides and polymeric supports, among others) Specifically, carbon supports have been frequently used in the catalysis field due to their specific properties such as acid and basic media resistance, thermal stability, metal recovery by combustion, low cost, and high flexibility in the pore structure and surface chemistry modification regarding the requirements of diverse catalytic chemical reactions.
The nature and concentration of surface functional groups is a relevant topic since it can strongly influence the anchoring of synthetic precursors or active phases in the synthesis of supported catalysts, or in a direct manner, they can act as active sites for catalytic reactions. Oxygenated functional groups (OFGs) such as carboxylate, carbonyl, phenol and lactone are the most relevant ones, since they can be spontaneously obtained by air exposure and its concentration can be modified by thermal and oxidative treatments [
1]. An increase in the surface OFG amount can be induced by treatment with oxidizing agents such as HNO
3, H
2O
2, H
2SO
4 and citric acid. Among them, HNO
3 shows the best performance, probably associated with its strong acid nature and high oxidizing potential. The degree of functionalization depends on the acid used, temperature, acid concentration and treatment time [
2]. One of the actual goals in the catalysis field is the design of selective-low-cost catalysts. In the particular case of partial oxidation reactions, noble-metal based systems have shown the best results [
3]. However, their high cost has focused attention on transition metals and transition metal oxides-based systems, such as Cu and Ni ones, with no appreciable conversion decreases [
4]. Particularly, copper has been applied to C-H breaking chemical reactions such as partial or selective oxidation reactions.
In this paper, monometallic Cu/ACx catalysts were synthesized and characterized in order to obtain information and a better understanding regarding the effects of the presence and nature of OFGs on the content and dispersion of surface copper species in AC and ACx samples. Moreover, the structural and surface chemical properties of the ACx samples were studied and correlated with the operational parameters of the functionalization process.
3. Results and Discussion
Since carbonous materials tend to decompose, in the presence of oxygen, at different temperatures depending on their crystal structure, and taking into account subsequent synthesis steps (decomposition of metallic precursors), thermal stability was tested in flowing air by TGA-DSC. TGA-DSC curves showed a first weight loss of 5% between 38–70 °C associated with surface water evaporation, and a second exothermic event between 460–665 °C corresponding to the thermal decomposition of AC (90% of the initial weight loss) [
5]. A limit temperature of 600 °C was selected for thermal treatments in a non-oxidizing atmosphere and to carry out the catalyst synthesis.
Surface functionalization was carried out using acids of different strengths. Two temperatures were tested for HNO3 (80 and 90 °C) and one temperature for citric acid (60 °C), in order to study the degree of functionalization and the distribution of the different surface species. Both effects may induce changes in structure and surface properties of the AC, particularly specific surface area (SBET) and surface acid-base properties.
XRD patterns of the samples are shown in
Figure 1A, with no appreciable changes in the structure of the AC support after functionalization. Acid-treated samples show a drop in the specific surface area (S
BET) of 20.6% for AC80, 16.3% for AC90 and 4.8% for ACC. These drops seem to be related with the possible formation of OFG species at the pore opening region, blocking the access to N
2 molecules [
6]. The S
BET decrease shows a direct correlation with the strength of the acid and the functionalization temperature.
Figure 1B shows the FT-IR spectra collected for the supports. A preliminary analysis suggests the presence of similar surface OFGs with some intensity discrepancies. The spectra show a band between 3100–3500 cm
−1 associated with the O-H stretching mode in hydroxyl, carboxyl and phenol groups; the band located at ≈ 2100 cm
−1–2250 cm
−1 is attributed to the alkyne group (C≡C), the bands located at 1636 cm
−1 and 1398–1100 cm
−1 could be assigned to the C=O and C-O stretching modes, respectively, of carboxyl, lactone, anhydride and ester OFGs. Finally, the signals observed at 670 cm
−1 were associated with C-OH torsion modes [
7]. All this information demonstrates the actual occurrence of the surface functionalization at different extents, depending on the conditions of the treatment.
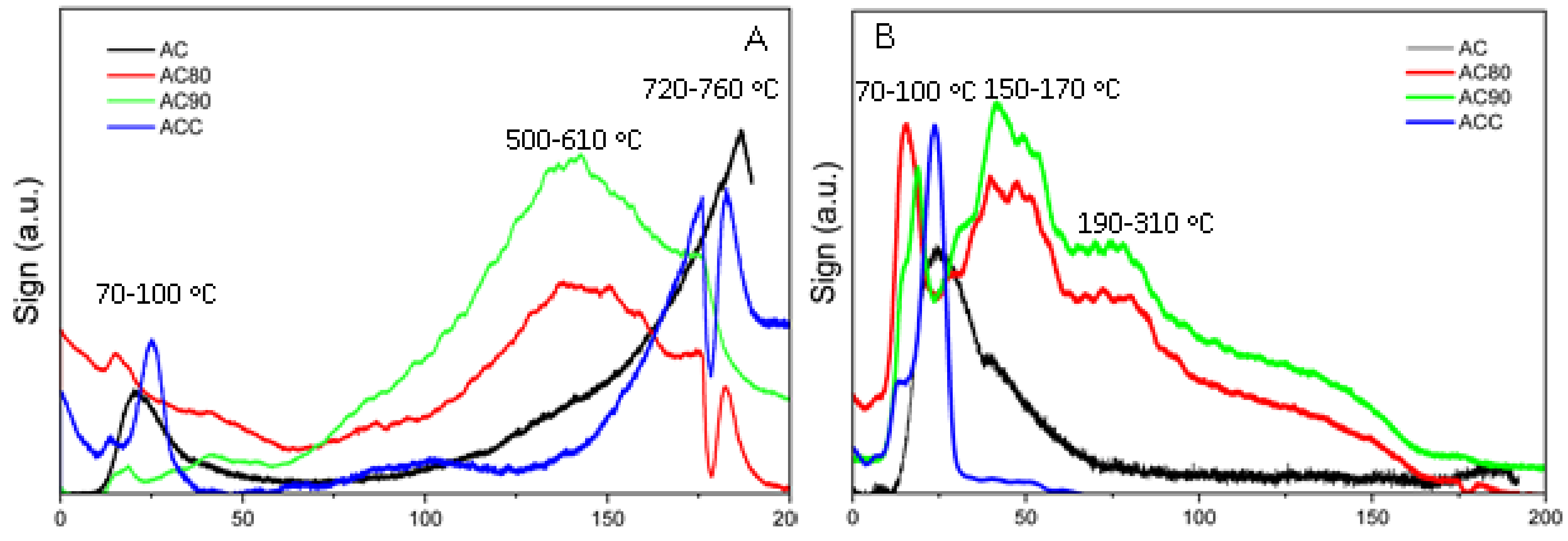
Support TPD measurements can be qualitatively and semi-quantitatively correlated with the surface OFG composition. For the case of the carbonous supports,
Figure 2 shows the evolution of CO and CO
2 signals associated with the thermal decomposition of the OFGs [
8]. The CO signal evolution is shown in
Figure 2A and is mainly associated with the high temperature decomposition of phenol, anhydride, quinone, and carbonyl surface groups, while
Figure 2B shows two different groups of CO
2 release-signals, the low temperature ones associated with the decomposition of carboxylic and anhydride groups and the high temperature ones with lactone decomposition. It has been reported that the decomposition of anhydride and carboxylic groups tends to generate both CO and CO
2 signals while phenol, ether, carbonyl and quinone group decomposition presents only CO signals of markedly higher intensities than the CO
2 ones observed for the other OFGs [
7].
In order to study semi-quantitatively the OFG surface content, the obtained signals shown in
Figure 3 were integrated after normalization considering the surface area of each sample (see
Table 1). The OFG amount increases with the strength of the acid treatment and temperature, showing the order AC < ACC < AC80 < AC90. The analysis of the integrated intensities calculated for the CO and CO
2 signals gives information about the OFG nature. By this means, the AC surface seems to be mainly composed of carboxyl and anhydride groups, while the HNO
3-treated samples showed a markedly higher CO signal intensity, suggesting a preferential functionalization to phenol, ester, carbonyl, and quinone OFGs. For the ACC sample, the observed CO
2/CO signal ratio shows a tendency to the generation of carboxyl and anhydride OFGs when citric acid is used [
7].
FTIR and TPD results probe the effective surface functionalization of the commercial AC and a clear dependency of this process on acid strength and temperature. The presence of different OFGs at the surface of the samples could affect the acid-base surface properties. In order to study this effect, the model reaction of dissociative adsorption of isopropanol (2-POH) was tested for the supports. This reaction allows the identification and quantification of different surface acid and basic sites, where depending on the nature of the surface sites the reaction can occur via two different paths: Dehydration or dehydrogenation of 2-POH [
9]. The first one occurs at the acid sites of the surface via an E1 and/or E2 mechanisms rendering propylene (P) and/or di-isopropyl-ether (DIE); whereas the second reaction occurs over basic and/or redox sites, rendering acetone (A) as a final product. Thus, the analysis of the obtained products provides helpful information about the density, strength and number of the sites at the surface [
10].
Figure 3 shows the results obtained for the 2-POH model reaction, where the AC support results show only a small amount of strong acid Lewis sites or strong basic Bronsted sites. Oxidative treatment modifies the acid and/or basic sites distribution. AC80 and AC90 show high basic sites content, being smaller in the case of AC90; moreover, the acid sites show a similar increase with respect to AC. In the case of ACC, the surface site distribution changes, displaying a high acid site content (similar to that observed for AC80 and AC90) and a minimum number of basic ones, manifesting the weak character of citric acid. From this analysis, the surface functionalization of the commercial AC is clear, generating different kinds and contents of surface OFGs, which can be traduced in a modified metal-support interaction.
Figure 1A displays the XRD patterns collected for the supports and catalysts. Two broad peaks centered at 2θ = 23 and 43° are due to the carbon support material. New thin peaks are observed at 2θ = 43.3, 50.5 and 74.3° for Cu/AC80 and Cu/AC90 corresponding to the main reflections of metallic copper (PDF: 96-901-3015), while a tiny reflection due to the presence of a small amount of Cu
2O is observed at 2θ = 36.4° (PDF: 96-900-5770) [
11]. Previously, the presence of reduced copper species in functionalized carbons due to OFG-mediated chemical reduction in the region next to the copper oxide in inert atmospheres and at high temperature was reported [
11]. On the other hand, AC and ACC show no evidence of reduced copper species in the XRD patterns, possibly related with the high dispersion and low percentage of the surface metal compounds.
The copper content of the catalysts was measured by AA spectroscopy and the results were 0.78%, 1.71%, 2.41% and 1.84% for Cu/AC, Cu/AC80, Cu/AC90 and Cu/ACC, respectively. From these data, a direct correlation between copper and surface OFGs contents can be proposed, where the Cu/AC sample shows the lowest OFG and copper content, while AC90 displays the highest OFG and copper content. Regarding ACC and AC80, both show intermediate content of copper and OFGs (TPD data from
Table 1). Taking into account the measured data for XRD and AAS, no intense XRD reflections should be expected in the four patterns. Cu/AC and Cu/ACC are in keeping with this expectation, showing no intense peaks associated with copper phases; however, both AC80 and AC90 displayed sharp reduced copper-related reflections. The intensity of these reflections (related with Cu°) are not related with a high copper content, but probably related with the amount and kind of surface OFGs, which under inert atmospheric treatments may reduce the Cu(II) species, rendering Cu° and Cu(I) ones. The small observed value of FWHM (0.22°) calculated for the peaks associated with Cu° and the coincidence of the d values (2.07 Å) measured for the (002) and (111) reflections of carbon and Cu°, respectively, suggests the possibility of an epitaxial growth of the metal on the (002) surface lattice plane of carbon, generating by this means “highly crystalline” reflections, even though its concentration is less than 2%. In fact, a comparison of the inter-atomic distances of Cu° and carbon at the above-mentioned crystal directions matches very well, supporting the epitaxial growth of Cu nanocrystals hypothesis. The presence of OFGs facilitates this metal growth, so interactions between copper and the OFGs seem to be of great importance in these systems. If this fact is confirmed, more information about the location of OFGs at the carbon surface and their interactions with copper species could be obtained, but at the moment this issue remains under debate.
SEM-EDS micrographs (
Figure 4), show a homogeneous distribution of copper species at the support surface, with no evidence of Cu-agglomerates for the Cu/AC80 and Cu/AC90 samples, supporting the hypothesis of the preferential orientation. Metallic dispersion and average metallic particle diameter were determined from N
2O dissociative adsorption experiments, as reported elsewhere [
12]. The calculated dispersion and average particle diameter for the catalysts were 6% (15.1 nm), 30% (3.3 nm), 22% (4.5 nm) and 17% (5.8 nm) for Cu/CA, Cu/AC80, Cu/AC90 and Cu/ACC, respectively. It can be seen that average values of small Cu metal domain sizes are obtained in the catalysts with the functionalized AC. This can be associated with different degrees of metal-support interaction, particularly with the type and content of OFGs evidenced by the larger Cu particle size of the Cu/AC catalyst, wherein the AC has not been functionalized.
At the moment, the catalysts Cu/ACx are being analyzed by XPS in order to determine the surface copper distribution, oxidation state and metal-support interaction. Furthermore, the ratio Cu2+/Cu+ and preferential copper environments are under study by EPR. In the next step, all these samples will be tested in the glycerol selective oxidation reaction.