1. Introduction
The consumption of antibiotics has grown substantially since their discovery, and they represent an important class of pharmaceuticals employed for treating bacterial infections by killing bacteria or preventing them from spreading. Antimicrobial drugs are widely utilized in human and veterinary medicine as well as in agriculture [
1]. Although these chemicals allow us to live longer and have healthier lives, their overconsumption poses a great threat, mainly regarding the development of antibiotic resistance [
2].
Clarithromycin is a macrolide, a class of antibiotic drugs produced by multiple
streptomyces strains, mostly effective against Gram-positive bacteria [
3]. It is paramount to understand the occurrence and fate of macrolides in the environment, since traces of these drugs are frequently detected in surface and ground waters. Accordingly, the European Commission included clarithromycin, together with other macrolides, in the 2nd Watch List of Emerging Water Pollutants. This surface water watch list was developed with the purpose of obtaining high-quality monitoring data regarding several potential water pollutants in order to establish their environmental and health risks, thus emphasizing the importance of developing efficient techniques to detect and quantify such pollutants.
Analytical methods based on liquid chromatography (LC) and mass spectrometry (MS) provide low detection limits but require intensive sample preparation, expensive equipment, and experienced operators and are not designed for in situ analysis [
4,
5]. Sensors are considered the future of monitoring tools and present many advantages compared to traditional techniques [
6]. Sensor devices are low cost, simple to operate, and can be used for continuous, fast, and reliable in situ monitoring [
7,
8,
9]. Even though sensors are not as selective as LC/MS methods, they have proved to be able to identify multiple analytes simultaneously [
6]. Moreover, sensors have the ability to work as smart devices that may be incorporated in monitoring systems with real-time data transmission.
The electronic tongue (e-tongue) refers to a device that consists of an array of non-specific chemical and/or physical sensors that display cross-sensitivity to target compounds in a liquid matrix [
10]. E-tongue devices may rely on potentiometry, voltammetry, or impedance spectroscopy as transducing methods [
11].
The present work aims to explore the potential of the e-tongue concept to monitor different clarithromycin concentrations in two environmental aqueous matrices with incremental complexity. Relying on the discussion of reproducibility, an array of sensors coated with PEI/PSS thin films, which previously showed more mechanical stability [
12], was studied as a potential smart device to monitor clarithromycin in mineral and surface water.
2. Materials and Methods
Clarithromycin (Sigma-Aldrich, Steinheim, Germany) solutions with concentrations between 10−15 M and 10−5 M were prepared by sequential dilutions of a mother solution with a concentration of 10−4 M. All dilutions, as well as the mother solution, were prepared with experimental matrix/MeOH (9:1) solutions. The experimental matrices utilized to prepare the solutions were a commercial Portuguese mineral water (MW) and a surface water (SW) collected from Tagus River at Porto Brandão, Caparica, Portugal. Lastly, solutions without clarithromycin, containing only experimental matrix/MeOH (9:1), were prepared for the MW and SW matrices to be used as the blank standard (0 M).
The sensors used in this work consist of interdigitated gold electrodes (IDE) deposited on ceramic and glass BK7 solid supports purchased from DropSens (Oviedo, Asturias, Spain). The dimensions of the ceramic support are 22.8 mm × 7.6 mm × 1 mm; the width of each “finger” and the spacing between “fingers” are both 200 μm. The glass support’s dimensions are 22.8 mm × 7.6 mm × 0.7 mm, and the width of each “finger”, like the spacing between “fingers”, is 10 μm. The sensor devices were coated with thin films of polyethyleneimine (PEI) and poly(sodium 4-styrenesulfonate) (PSS) produced by the layer-by-layer (LbL) technique [
13]. The substrates were alternately immersed in positively and negatively charged polyelectrolyte solutions with concentrations of 10
−2 M for 30 s. After the adsorption of each layer, the substrates were immersed in water in order to remove any polyelectrolyte molecules that were not completely adsorbed. At the end of the deposition of each bilayer, the substrates were dried with nitrogen gas stream (99% purity, Air Liquide, Algés, Portugal). The thin films of PEI/PSS were prepared with 5 bilayers, (PEI/PSS)
5. Prior to the deposition of thin films, all sensors were cleaned with ethanol and ultra-pure water. Thereafter, the substrates were dried with compressed nitrogen gas (99% purity, Air Liquide, Algés, Portugal).
The electrical analysis was achieved by impedance spectroscopy measurements of the sensor devices when immersed in aqueous matrices spiked with a sequence of increasing concentrations of clarithromycin, from 0 to 10−5 M. The impedance spectra were obtained with a Solartron 1260 Impedance Analyzer (Solartron Analytical, AMETEK Scientific Instruments, Berwyn, PA, USA) in the frequency range of 1 Hz to 1 MHz by applying an alternate voltage with an amplitude of 25 mV. Each measurement was performed at room temperature (≈23 °C).
Principal component analysis (PCA) was performed, with respect to the normalized (Z-score normalization: , and being the mean value and the standard deviation of the samples, respectively) impedance spectroscopy data, to reduce the size of the data and to obtain a new space of orthogonal components in order to detect and explain different concentration patterns. The clarithromycin detection in the target matrices was further evaluated by an array of sensors, composed of all of the produced thin films, using the e-tongue concept.
3. Results and Discussion
3.1. Sensor Reproducibility
To draw conclusions on the reproducibility of the (PEI/PSS)
5 sensors, each solution of clarithromycin was analyzed with two identical sensors produced under the same conditions. The average of the two sensors’ data was calculated as a function of the frequency for each matrix and type of sensor (glass or ceramic support). The standard deviation was used as a measure of the uncertainty. In
Figure 1 is depicted the average of the impedance magnitude measured by (a) two ceramic support and (b) two glass support sensors when immersed in the MW matrix.
Figure 1a reveals that the ceramic support sensors coated with (PEI/PSS)
5 are reproducible when monitoring clarithromycin in MW, since the values of the standard deviation are small (two orders of magnitude smaller than the average), meaning that the results of both sensors are similar. Furthermore,
Figure 1b shows that there is a significant discrepancy between the measurements of the two glass support sensors, which results in larger standard deviation values. These results can be explained, since the spacing between the IDE “fingers” is smaller in the glass support sensors, and, thus, the electric field generated between them, when an AC voltage signal is applied, has a larger magnitude. Additionally, the MW matrix has a lower conductivity, containing ion species in lower concentrations and affecting, therefore, the interactions between the matrix and the sensor. Consequently, at lower frequency values (<10 Hz), measurements of the glass sensors immersed in MW are compromised (see
Figure 1b, region highlighted by a red dashed rectangle).
Regarding the analysis of clarithromycin solutions prepared with SW,
Figure 2a,b shows the average of the capacitance measured by two ceramic support sensors and two glass support sensors, respectively.
Figure 2a reveals that there is not a considerable difference between the results of both ceramic sensors. As stated before, this conclusion can be drawn from the low values of the standard deviation (10
−10–10
−8 F).
Figure 2b provides similar conclusions; thus, both types of sensors show good reproducibility when monitoring SW matrices. However, for the SW matrix, the glass support sensors coated with (PEI/PSS)
5 thin films present a more noticeable sensitivity in discriminating clarithromycin concentrations in the frequency range of 1–100 Hz.
On the other hand, the sensors with a ceramic support showed superior reproducibility for both experimental matrices. Furthermore, the ceramic sensors produced more reliable results in the frequency region of 1–100 Hz for MW. For SW, both types of sensors achieved better reproducibility in the measurements from 1 Hz to 100 Hz.
3.2. Principal Component Analysis: E-Tongue
The results of the principal component analysis applied to the e-tongue concept will be presented and discussed only for SW. The e-tongue concept was not applied for MW, since the glass support sensors were not reproducible, as evidenced by the analysis of the impedance electrical characterization (
Figure 1b). Thus,
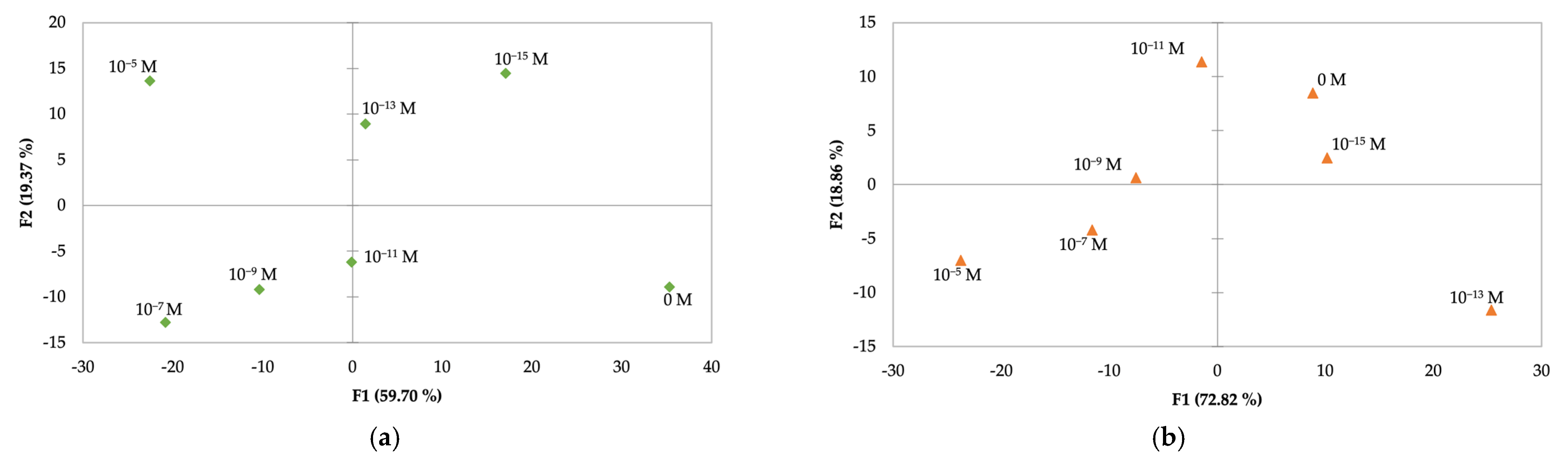
Figure 3 displays the PCA plots obtained for an array of sensors composed of two ceramic support sensors, for MW (
Figure 3a), and two ceramic support sensors combined with two glass support sensors for SW (
Figure 3b). In both target matrices, the array of sensors was coated with (PEI/PSS)
5 thin films.
In
Figure 3a, the first two principal components account for 91.68% of the total variance. The PCA plot reveals that the ceramic support sensors provide the ability to discriminate between different clarithromycin concentrations and the blank solution. There can also be observed a pattern in the concentration decay, with 10
−13 M as an outlier.
Figure 3b shows the PCA plot for an array of sensors identical to the one discussed above but immersed in SW solutions. In this case, the first two principal components accounted for 79.07% of the total variance. The plot reveals that the e-tongue concept provides the ability to discriminate between non-doped and doped SW solutions. Additionally, the sensors were able to distinguish the different clarithromycin concentrations.
4. Conclusions
Sensors composed of ceramic or glass BK7 solid supports, with interdigitated gold electrodes, were coated with five bilayers of PEI/PSS thin films produced by the LbL technique. An electronic tongue consisting of an array of these sensors was shown to provide the ability to distinguish between clarithromycin concentrations in the range of 10−15 M to 10−5 M in surface water.
The electrical analysis of the samples was performed with impedance spectroscopy by immersing the sensors in the water samples with different clarithromycin concentrations. An average of the measurements obtained with two identical sensors and the associated standard deviation were used to study the reproducibility of the sensors. In the MW matrix, the ceramic sensors showed reproducibility. The opposite can be said for the glass support sensors, which for lower frequencies struggle to identify the target compound. In the SW matrix, both types of sensors, ceramic or glass support, were proven to be highly reproducible.
Results of the principal component analysis of the impedance data did not show a clear pattern or trend but was able to distinguish between doped and non-doped solutions, both for MW and SW matrices. To achieve better results, the e-tongue concept requires a wider variety of thin films deposited on the sensors, such as, for example, metal oxides or carbon-based thin films.
Author Contributions
Conceptualization, C.M., T.M., M.R. and S.S.; methodology, T.M.; software, T.M.; validation, C.M., M.R. and S.S.; formal analysis, C.M, M.R. and S.S.; investigation, T.M. and C.M.; resources, P.A.R., M.R. and S.S.; data curation, T.M.; writing—original draft preparation, C.M., T.M., M.R. and S.S.; writing—review and editing, C.M., M.R. and S.S.; supervision, C.M., M.R. and S.S.; project administration, M.R.; funding acquisition, P.A.R., M.R. and S.S. All authors have read and agreed to the published version of the manuscript.
Funding
The research leading to these results has received funding from the Portuguese funding agency FCT—Fundação para a Ciência e a Tecnologia—within projects PTDC/FIS-NAN/0909/2014, UID/FIS/04559/2020 to LIBPhys-UNL from the FCT/MCTES/PIDDAC and the Bilateral Project entitled “Deteção de Estrogénio- um Contaminante Emergente em Corpos Hídricos” within the scope of “Cooperação Transnacional_FCT (Portugal)-CAPES (Brazil) 2018”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
C. Magro acknowledges NOVA.ID of NOVA-FCT for her post-doc fellowship.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Silbergeld, E.K.; Graham, J.; Price, L.B. Industrial food animal production, antimicrobial resistance, and human health. Annu. Rev. Public Health 2008, 29, 151–169. [Google Scholar] [CrossRef] [PubMed]
- Zimdahl, R.L. Antibiotics. In Six Chemicals That Changed Agriculture; Elsevier: Amsterdam, The Netherlands, 2015; pp. 165–182. [Google Scholar]
- Hagren, V.; Peippo, P.; Lövgren, T. Detecting and controlling veterinary drug residues in poultry. In Food Safety Control in the Poultry Industry; Mead, G.C., Ed.; Woodhead Publishing Limited: Southston, UK, 2005; pp. 44–82. [Google Scholar]
- Zhang, Y.; Zhu, Y.; Zeng, Z.; Zeng, G.; Xiao, R.; Wang, Y.; Hu, Y.; Tang, L.; Feng, C. Sensors for the environmental pollutant detection: Are we already there? Coord. Chem. Rev. 2021, 431, 213681. [Google Scholar] [CrossRef]
- Rawtani, D.; Khatri, N.; Tyagi, S.; Pandey, G. Nanotechnology-based recent approaches for sensing and remediation of pesticides. J. Environ. Manag. 2018, 206, 749–762. [Google Scholar] [CrossRef] [PubMed]
- Magro, C.; Mateus, E.P.; Fátima Raposo, M.; Ribeiro, A.B. Emerging Contaminants in Wastewater: Sensor Potential for Monitoring Electroremediation Systems. In Electrokinetic Remediation for Environmental Security and Sustainability; Wiley: Hoboken, NJ, USA, 2021; pp. 413–432. [Google Scholar]
- Ciosek, P.; Wróblewski, W. Sensor arrays for liquid sensing- electronic tongue systems. Analyst 2007, 132, 963–978. [Google Scholar] [CrossRef] [PubMed]
- Kruss, S.; Hilmer, A.J.; Zhang, J.; Reuel, N.F.; Mu, B.; Strano, M.S. Carbon nanotubes as optical biomedical sensors. Adv. Drug Deliv. Rev. 2013, 65, 1933–1950. [Google Scholar] [CrossRef]
- Barsan, M.M.; Ghica, M.E.; Brett, C.M.A. Electrochemical sensors and biosensors based on redox polymer/carbon nanotube modified electrodes: A review. Anal. Chim. Acta 2015, 881, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Vlasov, Y.; Legin, A. Non-selective chemical sensors in analytical chemistry: From “electronic nose” to “electronic tongue”. Fresenius J. Anal. Chem. 1998, 361, 255–260. [Google Scholar] [CrossRef]
- Latha, R.S.; Lakshmi, P.K. Electronic tongue: An analytical gustatory tool. J. Adv. Pharm. Technol. Res. 2012, 3, 3–8. [Google Scholar]
- Magro, C.; Zagalo, P.; Pereira-da-Silva, J.; Pires Mateus, E.; Branco Ribeiro, A.; Ribeiro, P.; Raposo, M. Polyelectrolyte Based Sensors as Key to Achieve Quantitative Electronic Tongues: Detection of Triclosan on Aqueous Environmental Matrices. Nanomaterials 2020, 10, 640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, O.N.; Raposo, M.; Dhanabalan, A. Langmuir-blodgett and self-assembled polymeric films. In Handbook of Surfaces and Interfaces of Materials; Nalwa, H.S., Ed.; Elsevier: Burlington, VT, USA, 2001; pp. 1–63. ISBN 978-0-12-513910-6. [Google Scholar]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).