Abstract
Illicit drug consumption is posing critical concerns in our society causing health issues, crime-related activities and the disruption of the border trade. The smuggling of illicit drugs urges the development of new tools for rapid on-site identification in cargos. Current methods used by law enforcement offices rely on presumptive color tests and portable spectroscopic techniques. However, these methods sometimes exhibit inaccurate results due to commonly used cutting agents or because the drugs are smuggled (hidden or mixed) in colored samples. Interestingly, electrochemical sensors can deal with these specific problems. Herein, it is presented an electrochemical device that uses low-cost screen-printed electrodes for the electrochemical detection of illicit drugs by square-wave voltammetry (SWV) profiling. A library of electrochemical profiles is built upon pure and mixtures of illicit drugs with common cutting agents. This library allows the design of a tailor-made script that shows the identification of each drug through a user-friendly interface. Finally, the results obtained from the analysis of different samples from confiscated cargos at an end-user laboratory present a promising alternative to current methods offering low-cost and rapid testing in the field.
1. Introduction
The consumption of drugs of abuse is causing critical issues in our society due to health issues, crime-related activities and the disruption of the border trade [1]. These illicit drugs can enter the illegal market through external borders (e.g., natural drugs) or by internal production (e.g., synthetic drugs). The smuggling of illicit drugs such as cocaine and heroin in Europe urges the development of new tools for rapid on-site identification in cargos. Besides, the production of synthetic drugs increases internal trafficking, thus demanding simple and straightforward devices to detect illicit drugs in the field. Current methods used by law enforcement offices rely on presumptive color tests [2] and portable spectroscopic techniques (e.g., near-infrared [3] and Raman spectroscopy [4]). However, these methods sometimes exhibit inaccurate results due to commonly used cutting agents or because the drugs are colored. Besides, drug traffickers are generating innovative ways to overcome traditional detection methods such as mixing with conventional goods (e.g., charcoal, food) or adding colorants or other substances to avoid the on-site determination by current methods. Therefore, new devices that can overcome the current problems are necessary to cope with the determination of smuggled illicit drugs in common goods.
The devices for on-site analysis must be portable, low-cost and user-friendly in order to be implemented and used by law enforcement officers [5]. Electrochemical sensors can provide the aforementioned features, and importantly, they can deal with current challenges, providing more reliable results in comparison to commercially available devices [6,7]. In this direction, portable and wearable electrochemical sensors have been designed for the detection of illicit drugs in different configurations [8], including glove-based sensors [9]. The electrochemical approach is based on the characteristic electrochemical profile (EP) of each compound that reveals the electroactive moieties of the target compound. Following this strategy, cocaine [10], ketamine [11], heroin [12] and synthetic cathinones [13] have been detected by using low-cost screen-printed electrodes (SPEs). Amphetamine is a special case as it is not electroactive in the potential window of commercial carbon SPEs. Therefore, an in situ derivatization by employing 1,2-Naphthoquinone-4-sulphonic acid sodium salt allows for its electrochemical detection [14]. Overall, the most used illicit drugs can be determined by electrochemical methods at certain conditions.
Herein, we present an electrochemical device that uses low-cost SPEs for the electrochemical detection of illicit drugs by square-wave voltammetry (SWV) profiling. A pH strategy based on the profiling of each illicit drug using specific buffers allows detection of the most-encountered illicit drugs (i.e., cocaine, MDMA, heroin and amphetamine). Hence, the electrochemical interrogation of the illicit drugs exhibits the oxidation of the electroactive moieties in each drug at a certain potential, with the exception of amphetamine that uses an in situ derivatization to unravel its oxidation peak. A library of electrochemical profiles is built upon pure and mixtures of illicit drugs with common cutting agents. This library allows the design of a tailor-made script that shows the identification of each drug through a user-friendly interface. Finally, the results obtained from the analysis of different samples from confiscated cargos at different end-users sites present a promising alternative to current methods. Overall, the fast analysis of samples with a portable electrochemical device exhibits a straightforward on-site detection aiming to facilitate the tasks of law enforcement agents in the field, thus providing a more secure border management and a safer society.
2. Methods
2.1. Materials
Standards of D,L-amphetamine ∙ HCl, methamphetamine ∙ HCl, 3,4-methylenedioxymethamphetamine ∙ HCl (MDMA), cocaine ∙ HCl and heroin ∙ HCl, were purchased from Chiron AS, Trondheim, Norway. Standards of paracetamol, caffeine and creatine were provided by National Institute for Criminalistics and Criminology (NICC, Brussels, Belgium). Confiscated samples of amphetamine, MDMA, cocaine and heroin were also provided by the NICC. Analytical grade salts of potassium chloride, potassium phosphate, sodium borate, sodium bicarbonate, sodium acetate and potassium hydroxide were purchased from Sigma-Aldrich (Overijse, Belgium). 1,2-Naphthoquinone-4-sulphonic acid sodium salt (NQS) (>98 %) was purchased from Tokyo Chemical Industry Co., LTD., Tokyo, Japan.
2.2. Methods
Square wave voltammograms and cyclic voltammograms were recorded using a MultiPalmSens4 or EmStat Pico potentiostats (PalmSens, Houten, The Netherlands) with PSTrace/MultiTrace. Disposable ItalSens SPEs (PalmSens, Houten, The Netherlands), containing a graphite working electrode (Ø = 3 mm), a carbon counter electrode and a (pseudo) silver reference electrode were used for all measurements. The SWV parameters that were used: potential range of 0.0–1.4 V, frequency 10 Hz, 25 mV amplitude and 5 mV step potential. All the square wave voltammograms are background corrected using the PSTrace software.
Electrochemical tests were performed in 20 mM buffer solutions with 100 mM KCl by applying 60 μL of the solution onto the SPE. Phosphate buffer, acetate buffer and hydrogen carbonate buffer were used for the detection of cocaine and heroin, MDMA and amphetamine, respectively. Preanodized SPEs for heroin detection were performed by applying 1.5 V for 60 s in PBS solution at pH 7 by drop casting 60 μL on the SPE [12].
The composition of the confiscated samples was previously analyzed in the forensic laboratory at NICC with gas chromatography-mass spectrometry (GC-MS) to subsequently validate the electrochemical approach. Besides, the confiscated samples were also analyzed by a handheld Raman spectrometer (Bruker Bravo, Ettingen, Germany).
3. Results and Discussion
3.1. Electrochemical Profiling of Illicit Drugs
The electrochemical profiling is based on the interrogation by SWV of the target molecules which exhibit an oxidation process at a certain potential. Hence, cocaine oxidation peak might differ from heroin oxidation peak, showing the possibility to identify the target by the specific peak potential [15]. Fortunately, illicit drugs contain moieties which are electroactive at the potential window of carbon SPEs. These moieties are usually secondary or tertiary amines that allow its oxidation, thus showing a peak signal during the electrochemical scan by SWV. However, some illicit drugs share some moieties which can exhibit some overlap in the oxidation potential. In previous works, our research group has optimized the detection of illicit drugs by exploring certain conditions during the SWVs. For example, the anodic pretreatment to unravel the phenolic group oxidation of the 6-monoacetylmorphine (6-MAM) (a byproduct of heroin degradation at pH 12) [12], the cathodic pretreatment to avoid the suppressing effect of some adulterants [13] or the use of different pH (as some moieties are not oxidizable in certain pH at SPE) [11]. Besides, some illicit drugs such as amphetamine need a derivatization step to allow its electrochemical profiling employing low-cost carbon SPEs. In this case, a simple mixing step with NQS launches a chemical reaction to a product that is electroactive at the SPE [14]. An oxidation peak was observed at 1.15 V due to NQS oxidation at the carbon SPE. Since this occurs outside the potential window for illicit drugs (from 0.6 to 1.03 V) in pH 10 it will not affect their identification.
3.2. Generating the Library of Electrochemical Profiles
Figure 1 shows the SWVs of pure illicit drugs (i.e., cocaine, heroin, MDMA and amphetamine) at pH 5, pH 10, pH 12 and pH 12 using anodic pretreated SPEs to record the specific electrochemical profiles of the molecules at certain conditions. Figure 1A,B shows the differences between anodic pretreated SPEs. Although similar profiles were obtained, a clear peak separation occurred in the MDMA signal. The pH dependence on the oxidation of illicit drugs is dramatically shown in the analysis at pH 5, where only heroin and MDMA exhibited electroactivity (Figure 1C). This fact assists in the proper identification of the unknown sample by a simple dual pH test. Finally, the pH assessment clearly exhibits that amphetamine is not electroactive, and only after the derivatization step (Figure 1D), an oxidation peak appears.
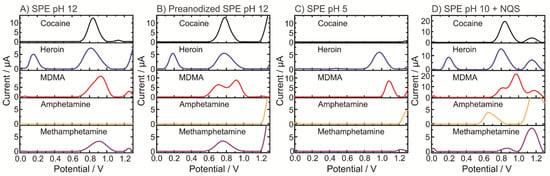
Figure 1.
Electrochemical profiles of illicit drugs (0.5 mM) obtained by square-wave voltammetry (SWV) using SPE at different pH: (A) pH 12; (B) pH 12 using preanodized SPE; (C) pH 5; and (D) pH 10 including the derivatizing agent NQS.
A similar electrochemical approach was performed employing the most encountered cutting agents (i.e., paracetamol, levamisole, lidocaine, caffeine, phenacetin, benzocaine, procaine and lactose [12,14,16]) (Figure 2A–D). Particularly interesting is the effect of the anodic pretreatment on the paracetamol signal exhibiting a sharper oxidation peak (Figure 2B). This permits the proper identification of the 6-MAM peak, thus avoiding peak overlap [12]. Besides, the effect of pH on the electrochemical signal showed a pH dependence as the oxidation peak shifts towards higher potentials at acidic pHs (Figure 2C). As pH 10 with NQS is targeted for the detection of amphetamine, only common cutting agents encountered in amphetamine real samples are explored (Figure 2D). Considering the profiling of the cutting agents, most of the peak potentials do not fall in the same position as the illicit drugs, thus allowing for a suitable identification in real samples.
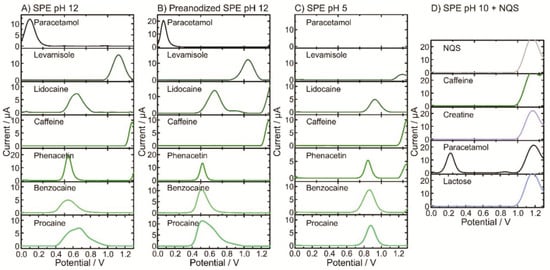
Figure 2.
Electrochemical profiles of common cutting agents (0.5 mM) obtained by square-wave voltammetry (SWV) using SPE at different pH: (A) pH 12; (B) pH 12 using preanodized SPE; (C) pH 5; and (D) pH 10 including the derivatizing agent NQS.
After building the library of electrochemical profiles with several conditions, a custom-made script (Matlab R2018b, MathWorks, Natick, MA, USA) is integrated. This script enhances the peak separation and facilitates the identification of the compounds in the sample. In brief, the script removes the background signal and applies a top-hat filter that provides an enhanced separation of overlapped peaks which permits a successful identification of the substances based on the peak potential of each drug. Therefore, the peak potential of each drug and cutting agent is introduced in the script to properly identify the drug and display it through a user-friendly interface.
3.3. Testing the Portable Electrochemical Device with Confiscated Samples
The electrochemical device consists of a miniaturized potentiostat with Bluetooth connectivity, a disposable SPE, a sampling container, a disposable spatula and a disposable pipette (Figure 3A). The sampling procedure consists of collecting the powder (either powder, liquid, crystal or impregnated material) with the disposable spatula into a tube containing 15 mL of the suitable buffer (Figure 2B). After shaking thoroughly, a drop of the solution is deposited on the SPE with the disposable pipette (Figure 2C). Subsequently, the operation is started on the user-friendly interface launching the electrochemical method, subsequent data treatment and results display (Figure 2D). For the analysis of confiscated samples, the strategies employing pH 12, preanodized SPE in pH 12, pH 5 and pH 10 with NQS were employed for cocaine, heroin, MDMA and amphetamine, respectively.
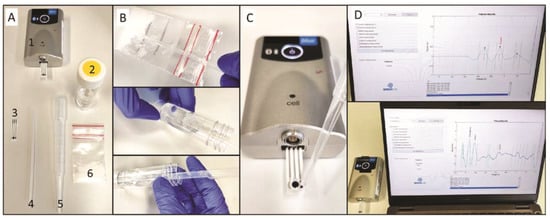
Figure 3.
On-site detection of illicit drugs with the portable electrochemical device. (A) Elements of the electrochemical device (1—potentiostat, 2—buffer container, 3—SPE, 4—disposable spatula, 5—disposable pipette, 6—confiscated sample); (B) Sampling procedure; (C) deposition of the solution on the setup ready for the electrochemical interrogation and (D) user-friendly interface showing the results of the analysis.
The reliability of the electrochemical device was evaluated in 40 confiscated samples provided by NICC (Table 1). After the analysis, the 40 samples were all positive for the corresponding illicit drug using the described sampling method in comparison to the standard methods (GC-MS). Besides, a portable Raman spectrometer was also used as a commonly used method in border settings. The electrochemical reader and portable Raman spectrometer exhibited an accuracy of 100% and 50%, respectively, calculated employing (observed detection by the method/actual detection by the GC-MS) × 100. Therefore, the electrochemical device outperformed the Raman device, particularly in heroin and amphetamine detection. It is worth mentioning that the low performance of the Raman device could be attributed to the colored nature of the samples, thus exhibiting one of the flaws of current methods. Overall, the electrochemical device is positioned as a reliable alternative for its use in the field due to its affordability, reliability and user-friendliness.

Table 1.
Results of the analysis by the analytical methods and composition of the confiscated samples.
4. Conclusions
In this work, the analysis of confiscated samples from illicit drugs is presented by the use of a portable electrochemical device. First, the construction of a library from several electrochemical profiles of standards of illicit drugs and common cutting agents at different conditions by SPE is performed. After the selection of the suitable conditions and the integration of the peak potentials of each target into a tailor-made script, the electrochemical device is ready for on-site analysis. The examination of 40 confiscated samples with the electrochemical device and a portable Raman spectrometer showed an outstanding performance of the electrochemical device in front of the Raman device according to the GC-MS identification. Overall, the electrochemical device based on SPE is presented as a promising alternative to current rapid and on-site methods for the detection of illicit drugs at border and coast controls.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/CSAC2021-10612/s1.
Author Contributions
Conceptualization, M.P.; methodology, M.P., A.S. and N.F.M.; software, R.V.E.; validation, M.P., A.S. and F.V.D.; M.P.; investigation, M.P.; resources, K.D.W. and F.V.D.; data curation, M.P. and A.S.; writing—original draft preparation, M.P.; writing—review and editing, M.P.; visualization, M.P.; supervision, K.D.W.; project administration, K.D.W.; funding acquisition, K.D.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by European Union’s Horizon 2020 research and innovation program, grant number 833787, Bordersens.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- European Monitoring Centre for Drugs and Drug Addiction. European Drug Report: Trends and Developments; European Monitoring Centre for Drugs and Drug Addiction: Lisbon, Portugal, 2020.
- Philp, M.; Fu, S. A review of chemical ‘spot’ tests: A presumptive illicit drug identification technique. Drug Test. Anal. 2018, 10, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Kranenburg, R.F.; Verduin, J.; Weesepoel, Y.; Alewijn, M.; Heerschop, M.; Koomen, G.; Keizers, P.; Bakker, F.; Wallace, F.; van Esch, A.; et al. Rapid and robust on-scene detection of cocaine in street samples using a handheld near-infrared spectrometer and machine learning algorithms. Drug Test. Anal. 2020, 12, 1404–1418. [Google Scholar] [CrossRef] [PubMed]
- Kranenburg, R.F.; Verduin, J.; Ridder, R.; Weesepoel, Y.; Alewijn, M.; Heerschop, M.; Keizers, P.H.J.; Esch, A.; Asten, A.C. Performance Evaluation of Handheld Raman Spectroscopy for Cocaine Detection in Forensic Case Samples. Drug Test. Anal. 2020, 13, 1054–1067. [Google Scholar] [CrossRef] [PubMed]
- de Araujo, W.R.; Cardoso, T.M.G.; da Rocha, R.G.; Santana, M.H.P.; Muñoz, R.A.A.; Richter, E.M.; Paixão, T.R.L.C.; Coltro, W.K.T. Portable analytical platforms for forensic chemistry: A review. Anal. Chim. Acta 2018, 1034, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Florea, A.; de Jong, M.; De Wael, K. Electrochemical strategies for the detection of forensic drugs. Curr. Opin. Electrochem. 2018, 11, 34–40. [Google Scholar] [CrossRef]
- De Rycke, E.; Stove, C.; Dubruel, P.; De Saeger, S.; Beloglazova, N. Recent developments in electrochemical detection of illicit drugs in diverse matrices. Biosens. Bioelectron. 2020, 169, 112579. [Google Scholar] [CrossRef] [PubMed]
- Teymourian, H.; Parrilla, M.; Sempionatto, J.R.; Montiel, N.F.; Barfidokht, A.; Van Echelpoel, R.; De Wael, K.; Wang, J. Wearable Electrochemical Sensors for the Monitoring and Screening of Drugs. ACS Sens. 2020, 5, 2679–2700. [Google Scholar] [CrossRef] [PubMed]
- de Jong, M.; Sleegers, N.; Kim, J.; Van Durme, F.; Samyn, N.; Wang, J.; De Wael, K. Electrochemical fingerprint of street samples for fast on-site screening of cocaine in seized drug powders. Chem. Sci. 2016, 7, 2364–2370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Jong, M.; Florea, A.; Eliaerts, J.; Van Durme, F.; Samyn, N.; De Wael, K. Tackling Poor Specificity of Cocaine Color Tests by Electrochemical Strategies. Anal. Chem. 2018, 90, 6811–6819. [Google Scholar] [CrossRef] [PubMed]
- Schram, J.; Parrilla, M.; Sleegers, N.; Samyn, N.; Bijvoets, S.M.; Heerschop, M.W.J.; van Nuijs, A.L.N.; De Wael, K. Identifying Electrochemical Fingerprints of Ketamine with Voltammetry and Liquid Chromatography–Mass Spectrometry for Its Detection in Seized Samples. Anal. Chem. 2020, 92, 13485–13492. [Google Scholar] [CrossRef] [PubMed]
- Felipe Montiel, N.; Parrilla, M.; Beltrán, V.; Nuyts, G.; Van Durme, F.; De Wael, K. The opportunity of 6-monoacetylmorphine to selectively detect heroin at preanodized screen printed electrodes. Talanta 2021, 226, 122005. [Google Scholar] [CrossRef] [PubMed]
- Schram, J.; Parrilla, M.; Sleegers, N.; Van Durme, F.; Van Den, J.; van Nuijs, A.L.N.; De Wael, K. Electrochemical profiling and LC-MS characterization of synthetic cathinones: From methodology to detection in forensic samples. Drug Test. Anal. 2021, 13, 1282–1294. [Google Scholar] [CrossRef] [PubMed]
- Parrilla, M.; Montiel, N.F.; Van Durme, F.; Wael, K. De Derivatization of amphetamine to allow its electrochemical detection in illicit drug seizures. Sens. Actuators B Chem. 2021, 337, 129819. [Google Scholar] [CrossRef]
- Moro, G.; Barich, H.; Driesen, K.; Felipe Montiel, N.; Neven, L.; Mendonça, C.D.; Shanmugam, S.T.; Daems, E.; Wael, K. De Unlocking the full power of electrochemical fingerprinting for on-site sensing applications. Anal. Bioanal. Chem. 2020, 412, 5955–5968. [Google Scholar] [CrossRef] [PubMed]
- Broséus, J.; Gentile, N.; Esseiva, P. The cutting of cocaine and heroin: A critical review. Forensic Sci. Int. 2016, 262, 73–83. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).