Synthesis and Leishmanicidal Activity of Molecular Hybrids 1,2,3-Triazole-Chalcones †
Abstract
:1. Introduction
2. Methods
2.1. General
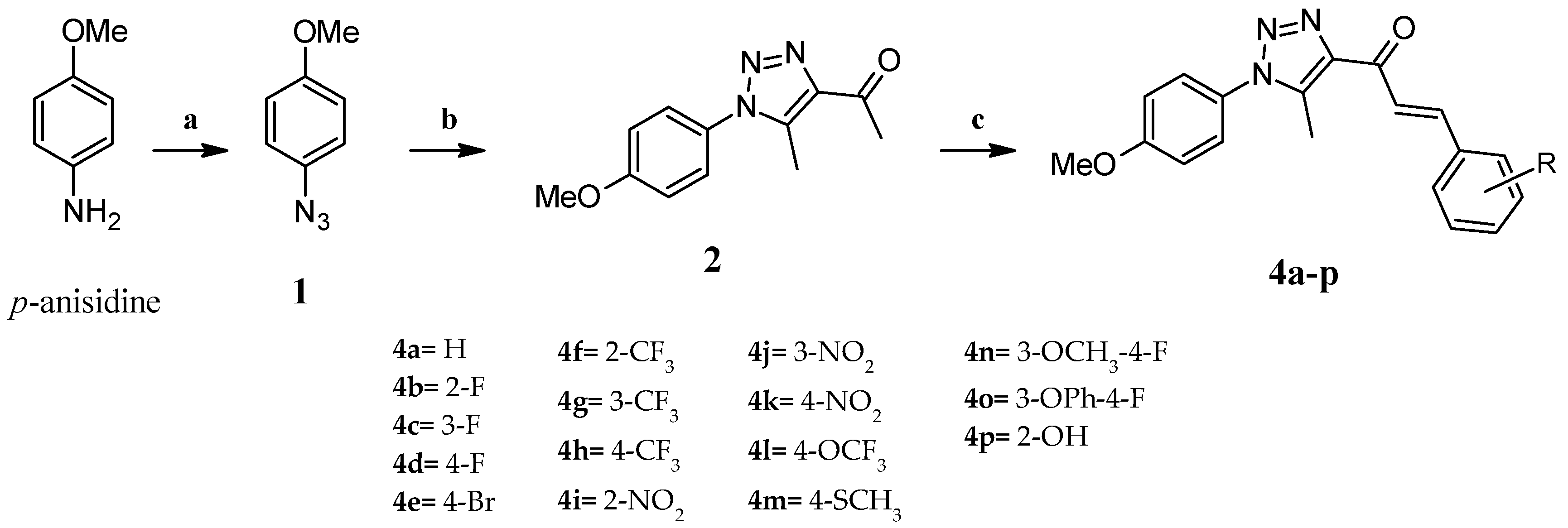
2.2. Synthesis of 1-Azido-4-methoxybenzene (1)
2.3. Synthesis of 1-[1-(4-Methoxyphenyl)-5-methyl-1H-1,2,3-triazol-4-yl]-ethanone (2)
2.4. General Procedure for the Synthesis of (2E)-1-[1-(4-Methoxyphenyl)-5-methyl-1H-1,2,3-triazol-4-yl]-3-arylprop-2-en-1-one Derivatives (4a–o)
2.5. Synthesis of (E)-1-[1-(4-Methoxyphenyl)-5-methyl-1H-1,2,3-triazol-4-yl]-3-(2-hydroxyphenyl)-prop-2-en-1-One (4p)
2.6. Evaluation of Leishmanicidal Activity
2.7. Evaluation of the Cell Viability
3. Results and Discussion
3.1. Synthesis
3.2. Biological Evaluation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Arenas, R.; Torres-Guerrero, E.; Quintanilla-Cedillo, M.R.; Ruiz-Esmenjaud, J. Leishmaniasis: A review. F1000 Rev. 2017, 6, 750. [Google Scholar]
- Braga, S.S. Multi-target drugs active against leishmaniasis: A paradigm of drug repurposing. Eur. J. Med. Chem. 2019, 183, 111660. [Google Scholar] [CrossRef] [PubMed]
- Akhoundi, M.; Kuhls, K.; Cannet, A.; Votýpka, J.; Marty, P.; Delaunay, P.; Sereno, D. A Historical Overview of the Classification, Evolution, and Dispersion of Leishmania Parasites and Sandflies. PLoS Negl. Trop. Dis. 2016, 10, e0004349. [Google Scholar] [CrossRef] [PubMed]
- Kobets, T.; Grekov, I.; Lipoldova, M. Leishmaniasis: Prevention, Parasite Detection and Treatment. Curr. Med. Chem. 2012, 19, 1443–1474. [Google Scholar] [CrossRef]
- World Health Organization. Leishmaniasis. Available online: https://www.who.int/en/news-room/fact-sheets/detail/leishmaniasis (accessed on 2 June 2020).
- Croft, S.L.; Sundar, S.; Fairlamb, A.H. Drug resistance in leishmaniasis. Clin. Microbiol. Rev. 2006, 19, 111–126. [Google Scholar] [CrossRef]
- World Health Organization. Control of the Leishmaniasis: Report of a Meeting of the WHO Expert Committee on the Control of Leishmaniases; WHO Press: Geneve, Switzerland, 2010; Volume 949, pp. 54–73. [Google Scholar]
- Gomtsyan, A. Heterocycles in drugs and drug discovery. Chem. Heterocycl. Compd. 2012, 48, 7–10. [Google Scholar] [CrossRef]
- Temraz, M.G.; Elzahhar, P.A.; Bekhit, A.E.D.A.; Bekhit, A.A.; Labib, H.F.; Belal, A.S.F. Anti-leishmanial click modifiable thiosemicarbazones: Design, synthesis, biological evaluation and in silico studies. Eur. J. Med. Chem. 2018, 151, 585–600. [Google Scholar] [CrossRef]
- Holla, B.S.; Mahalinga, M.; Karthikeyan, M.S.; Poojary, B.; Akberali, P.M.; Kumari, N.S. Synthesis, characterization and antimicrobial activity of some substituted 1,2,3-triazoles. Eur. J. Med. Chem. 2005, 40, 1173–1178. [Google Scholar] [CrossRef]
- Arlindo Pascual, Z.; Carrera González, S.; Francisco González Matilla, J. Synthesis of Chalcones: Privilegial Structures in the Synthesis of Heterocycles with Biological Activity. Psychol. Lat. Copyr. 2018, 20–23. [Google Scholar] [CrossRef]
- Viegas-Junior, C.; Danuello, A.; da Silva Bolzani, V.; Barreiro, E.J.; Fraga, C.A.M. Molecular Hybridization: A Useful Tool in the Design of New Drug Prototypes. Curr. Med. Chem. 2007, 14, 1829–1852. [Google Scholar] [CrossRef]
- Dyall, L.K.; Suffolk, P.M.; Dehaen, W.; L’Abbé, G. Factors affecting the rates of thermal decomposition of azidothiophenes. J. Chem. Soc. Perkin Trans. 2 1994, 10, 2115–2118. [Google Scholar] [CrossRef]
- Kharb, R.; Shahar Yar, M.; Sharma, P.C. New Insights into Chemistry and Anti-Infective Potential of Triazole Scaffold. Curr. Med. Chem. 2011, 18, 3265–3297. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.S.; Wang, H.C.; Gao, Z.L.; Li, R.S.; Cui, F.H. Tandem Michael addition/imino-nitrile cyclization synthesis of 2-amino-6-(1-aryl-5-methyl-1H-1,2,3-triazol-4yl)-4-phenylpyridine-3-carbonitrile. J. Heterocycl. Chem. 2010, 47, 389–395. [Google Scholar] [CrossRef]
- Li, J.T.; Yang, W.Z.; Wang, S.X.; Li, S.H.; Li, T.S. Improved synthesis of chalcones under ultrasound irradiation. Ultrason. Sonochem. 2002, 9, 237–239. [Google Scholar] [CrossRef]
- Nikzad, S.; Baradaran-Ghahfarokhi, M.; Nasri, P. Dose-response modeling using MTT assay: A short review. Life Sci. J. 2014, 11, 1097–8135. [Google Scholar] [CrossRef]
- Gorlushko, D.A.; Filimonov, V.D.; Krasnokutskaya, E.A.; Semenischeva, N.I.; Go, B.S.; Hwang, H.Y.; Cha, E.H.; Chi, K.W. Iodination of aryl amines in a water-paste form via stable aryl diazonium tosylates. Tetrahedron Lett. 2008, 44, 1243–1244. [Google Scholar] [CrossRef]
- Nelson, R.; Kesternich, V.; Pérez-Fehrmann, M.; Jaldin, S.; Marcourt, L.; Christen, P. Regiospecific synthesis of 1,4,5-trisubstituted 1,2,3-triazoles via enolate-azide cycloaddition between 1,3-dicarbonyl compounds and aryl azides. J. Chem. Res. 2016, 40, 453–457. [Google Scholar] [CrossRef]
- Perrin, C.L.; Chang, K.L. The Complete Mechanism of an Aldol Condensation. J. Org. Chem. 2016, 81, 5631–5635. [Google Scholar] [CrossRef]
- Daskiewicz, J.B.; Comte, G.; Barron, D.; Di Pietro, A.; Thomasson, F. Organolithium mediated synthesis of prenylchalcones as potential inhibitors of chemoresistance. Tetrahedron Lett. 1999, 40, 7095–7098. [Google Scholar] [CrossRef]
- Xin, Y.; Zang, Z.H.; Chen, F.L. Ultrasound-promoted synthesis of 1,5-diarylpenta-2,4-dien-1-ones catalyzed by activated barium hydroxide. Synth. Commun. 2009, 39, 4062–4068. [Google Scholar] [CrossRef]
- Jin, H.; Xiang, L.; Wen, F.; Tao, K.; Liu, Q.; Hou, T. Improved synthesis of chalconoid-like compounds under ultrasound irradiation. Ultrason. Sonochem. 2008, 15, 681–683. [Google Scholar] [CrossRef]
- Bui, T.H.; Nguyen, N.T.; Dang, P.H.; Nguyen, H.X.; Nguyen, M.T.T. Design and synthesis of chalcone derivatives as potential non-purine xanthine oxidase inhibitors. Springerplus 2016, 5, 1789. [Google Scholar] [CrossRef]
- Dolbier, W.R. An Overview of Fluorine NMR. In Guide to Fluorine NMR for Organic Chemists; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2008; pp. 9–34. [Google Scholar]

| Compound | R | Time (h) | Appearance/Color | M.p. (°C) | Yield 1 (%) |
|---|---|---|---|---|---|
| 4a | H | 8 | Crystals/White | 160.6–161.4 | 95.7 |
| 4b | 2-F | 5 | Crystals/White | 113.9–115.5 | 73.6 |
| 4c | 3-F | 5 | Crystals/White | 127.9–128.5 | 76.7 |
| 4d | 4-F | 5 | Crystals/White | 162.5–163.8 | 98.0 |
| 4e | 4-Br | 24 | Crystals/White | 159.1–160.2 | 96.4 |
| 4f | 2-CF3 | 24 | Crystals/White | 137.6–144.6 | 97.1 |
| 4g | 3-CF3 | 6 | Crystals/White | 140.5–142.9 | 77.5 |
| 4h | 4-CF3 | 6 | Crystals/White | 145.2–147.6 | 76.8 |
| 4i | 2-NO2 | 26 | Crystals/White | 193.6–195.5 | 33.5 |
| 4j | 3-NO2 | 6 | Crystals/Yellowish | 177.5–178.1 | 75.2 |
| 4k | 4-NO2 | 25 | Crystals/White | 231.7–236.9 | 96.7 |
| 4l | 4-OCF3 | 6 | Crystals/White | 128.5–129.7 | 88.3 |
| 4m 2 | 4-SCH3 | 8 | Crystals/White | 153.5–156.2 | 93.4 |
| 4n | 3-OCH3-4-F | 7 | Crystals/White | 158.3–159.6 | 96.1 |
| 4o | 3-OPh-4-F | 5 | Crystals/White | 108.0–109.8 | 99.3 |
| 4p 2 | 2-OH | 1 | Crystals/White | 195.6–196.9 | 99.6 3 |
| Compound | Leishmanicidal Activity IC50 (µM) | RAW Cytotoxicity CC50 (µM) | SI Index |
|---|---|---|---|
| 4a | 15.7 | 20.1 | 1.3 |
| 4b | 7.9 | 13.7 | 1.7 |
| 4c | 14.4 | 26.2 | 1.8 |
| 4d | NA | 44.3 | ND |
| 4e | NA | 22.4 | ND |
| 4f | NA | 23.2 | ND |
| 4g | 3.9 | 11.3 | 2.9 |
| 4h | 4.9 | 19.5 | 4.0 |
| 4i | NA | 4.6 | ND |
| 4j | 1.0 | 3.6 | 3.7 |
| 4k | ND | ND | ND |
| 4l | 27.0 | >100 | >3.7 |
| 4m | NA | ND | ND |
| 4n | NA | 16.2 | ND |
| 4o | 29.2 | 1.7 | 0.1 |
| 4p | 1.3 | 7.3 | 5.7 |
| Amphotericin B | 0.172 | >5 | ND |
| Saponin | ND | 0.163 * | ND |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Gutiérrez, S.V.; Barreiro-Costa, O.; León, C.D.A.; Heredia-Moya, J. Synthesis and Leishmanicidal Activity of Molecular Hybrids 1,2,3-Triazole-Chalcones. Chem. Proc. 2021, 3, 55. https://doi.org/10.3390/ecsoc-24-08356
Rodríguez-Gutiérrez SV, Barreiro-Costa O, León CDA, Heredia-Moya J. Synthesis and Leishmanicidal Activity of Molecular Hybrids 1,2,3-Triazole-Chalcones. Chemistry Proceedings. 2021; 3(1):55. https://doi.org/10.3390/ecsoc-24-08356
Chicago/Turabian StyleRodríguez-Gutiérrez, Sofía Vanessa, Olalla Barreiro-Costa, Christian David Alcívar León, and Jorge Heredia-Moya. 2021. "Synthesis and Leishmanicidal Activity of Molecular Hybrids 1,2,3-Triazole-Chalcones" Chemistry Proceedings 3, no. 1: 55. https://doi.org/10.3390/ecsoc-24-08356
APA StyleRodríguez-Gutiérrez, S. V., Barreiro-Costa, O., León, C. D. A., & Heredia-Moya, J. (2021). Synthesis and Leishmanicidal Activity of Molecular Hybrids 1,2,3-Triazole-Chalcones. Chemistry Proceedings, 3(1), 55. https://doi.org/10.3390/ecsoc-24-08356






