1. Introduction
Confocal laser scanning microscopy (CLSM) is a powerful technique most commonly used for in vitro and ex vivo research, and it includes a laser to scan the three-dimensional volume of an object point-by-point with a similar design to the scanning electron microscope. Moreover, CSLM combines the laser scanning method with fluorescence microscopy, using fluorophores that specifically target and identify subcellular structures: cytoplasm, sarcoplasmic reticulum, nuclei, and mitochondria. Thereby, CSLM has several advantages, such as sensitivity and specificity, by allowing better and sharper detection of the target, reducing background information away from the focal plane, ability to adjust the depth of field, and capability to collect various serial optical sections from thick specimens. In addition, conventional microscopy (light microscopy, SEM, TEM) has limitations due to sample staining and/or mechanical section, whereas confocal laser scanning microscopy enables researchers to obtain a three-dimensional image of nanoparticles distribution by way of optical sectioning.
Although nanoparticles have a great potential for cardiovascular therapy, there is increasing interest in nanoparticles’ safety and efficacy on human health [
1,
2,
3,
4,
5]. Despite various studies on penetration and mechanism of nanoparticles distribution in cells, the nanoparticles’ behavior remains sub judice with conflicting results reported [
6]. Physicochemical nanoparticle properties, formulation and environmental conditions are factors that affect their penetration through cells and therefore, they make it difficult to draw general conclusions on nanoparticle distribution [
7]. For this reason, we have investigated the intracellular uptake of polymeric nanoparticles loaded with two cardiovascular drugs (valsartan and amlodipine besylate) using modern fluorescence microscopy. It is well-known that nanoparticles can enter through cell membranes, often by endocytotic mechanisms. Herein we have focused on the penetration of polymeric nanoparticles across the plasma membranes of NIH3T3. By using CSLM, we have observed that nanoparticles penetrate through the cell membrane so that they can be found inside the cells.
2. Materials and Methods
2.1. Materials
Poly(D,L-lactide-co-glycolide) (PLGA, 50:50, MW = 30,000–60,000 Da), amlodipine besylate, valsartan and Pluronic F127 were purchased from Sigma-Aldrich Co. (Germany). Acetone (analytical grade) was obtained from AdraChim SRL (Bucharest, Romania) and used without further purification. The water used for all experiments was distilled. The fluorophores, Rhodamine B, Hoechst and phthalocyanine, were obtained from Sigma-Aldrich Co. (Germany). Adherent mouse embryo fibroblasts (NIH 3T3) were ATCC® CRL-1658™.
2.2. Preparation of PLGA NPs Loaded with Valsartan-Amlodipine
PLGA NPs loaded with valsartan-amlodipine were synthesized via the nanoprecipitation method according to previous reports [
8,
9]. Briefly, PLGA and cardiovascular drugs were dissolved in acetone (organic phase), while Pluronic-F127 was dissolved in distilled water (aqueous phase). The organic phase was added slowly dropwise into the aqueous phase solution and stirred magnetically at 1200 at room temperature until complete evaporation of the organic solvent. The final nanosuspension was centrifuged at 10,000 rpm for 30 min at 30 °C to separate the formed nanoparticles from the free drug. The nanoparticles were resuspended in distilled water polymeric aggregates and stored at 4 °C.
2.3. Cell Internalization Studies
The cells were seeded into a UV disinfected 6-well plate with cover glass. At designated incubation periods, nanoparticles stained with phthalocyanine were added into each well. At 48 h and 72 h, the suspension was removed, and the samples were washed with PBS solution three times, then mounted in fluorescent mounting medium to be observed by a confocal microscope Zeiss LSM710 with Zeiss PALM microdissection system.
3. Results and Discussion
To achieve the aim of this paper, the study of the intracellular uptake of polymeric nanoparticles loaded with cardiovascular drugs using CLSM, polymeric nanoparticles were prepared via nanoprecipitation method using poly(lactide-co-glycolide) (PLGA) as biodegradable polymeric matrix and Pluronic F127 as a stabilizer. A mixture of two cardiovascular drugs—valsartan (an angiotensin II receptor antagonist drug) and amlodipine besylate (a calcium channel blocker)—was loaded in polymeric nanoparticles. The formulation of PLGA NPs loaded with valsartan-amlodipine (PLGA:valsartan:amlodipine besylate = 10:16:1) was established in previous studies [
8,
9] as optimal having high EE (%) for both drugs (91.98 ± 0.18% for valsartan and 82.54 ± 0.12% for amlodipine besylate, nanometric particle size (140.4 ± 1.34 nm) and narrow dispersity (polydispersity index = 0.108 ± 0.03). The cellular uptake of polymeric nanoparticles was investigated by incubating adherent mouse embryo fibroblasts (NIH 3T3) with a suspension of PLGA NPs loaded with valsartan-amlodipine. The nanoparticles were previously stained with phthalocyanine. Targeted cell compartments were labeled with two fluorophores: rhodamine B (membrane stain) and Hoechst (nucleic acid stain). Live cell imaging was performed using a confocal microscope Zeiss LSM710 with Zeiss PALM microdissection system.
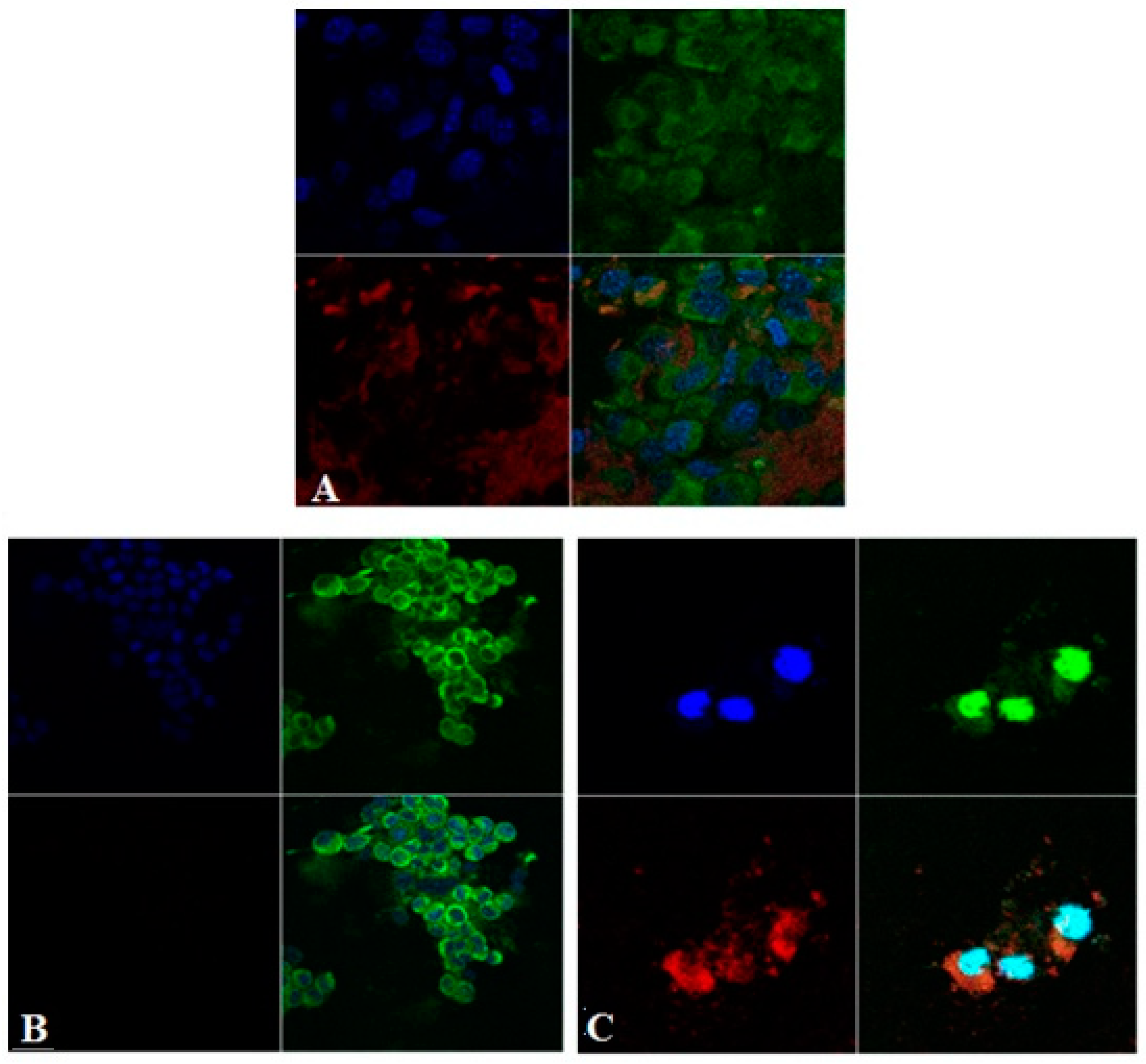
Figure 1 shows confocal microscopic images after exposure of NIH 3T3 cells to PLGA NPs. Green fluorescence is obtained from the green channel (excitation wavelength: 554 nm, emission wavelength: 627 nm), representing green stained cell membrane with rhodamine B. The blue fluorescence is from the blue channel (excitation wavelength: 350 nm, emission wavelength: 461 nm), which shows blue stained cell nucleic acid with Hoechst. The red fluorescence is from the red channel (excitation wavelength: 633, emission wavelength: 690 nm), which shows the red-stained PLGA NP with phthalocyanine. We were able to visualize the aggregates of nanoparticles in the extracellular space and especially the internalization of some smaller NP aggregates with a perinuclear intracytoplasmic localization.
Confocal microscopy images show an intracytoplasmic localization in vesicular formations surrounded by a membrane marked with rhodamine B. It cannot be detected whether NPs are included in cytoplasmic organs (endoplasmic reticulum, Golgi apparatus, lysosomes, peroxisomes, mitochondria only) located in vesicular endosomal formations. It is known that the main mechanism of incorporation of NP the process of endocytosis; however, it is not yet stated exactly whether it is just clathrin-mediated endocytosis or other types (caveolin-mediated endocytosis, independent clathrin and caveolin endocytosis, pinocytosis, etc.). NP enters through the membrane and correlating with the data in the literature. We took the first step in understanding the path of these nanosystems.
4. Conclusions
Polymeric nanoparticles loaded with valsartan-amlodipine were prepared via the nanoprecipitation method and had sizes lower than 300 nm and narrow dispersity. The cellular uptake of polymeric nanoparticles was investigated by incubating adherent mouse embryo fibroblasts with a suspension of nanoparticles stained previously with phthalocyanine. Targeted cell compartments were labeled with two fluorophores: Rhodamine B (membrane stain) and Hoechst (nucleic acid stain). Live cell imaging was performed by using confocal laser scanning microscopy. The intracellular uptake of polymeric nanoparticles was revealed by confocal laser scanning microscopy. The results suggest a possible mechanism of endocytosis and clearly a vesicular-based accumulation of the nanoparticles in the cytoplasmatic compartments.