Improved Catalytic Transfer Hydrogenation of Levulinate Esters with Alcohols over ZrO2 Catalyst †
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
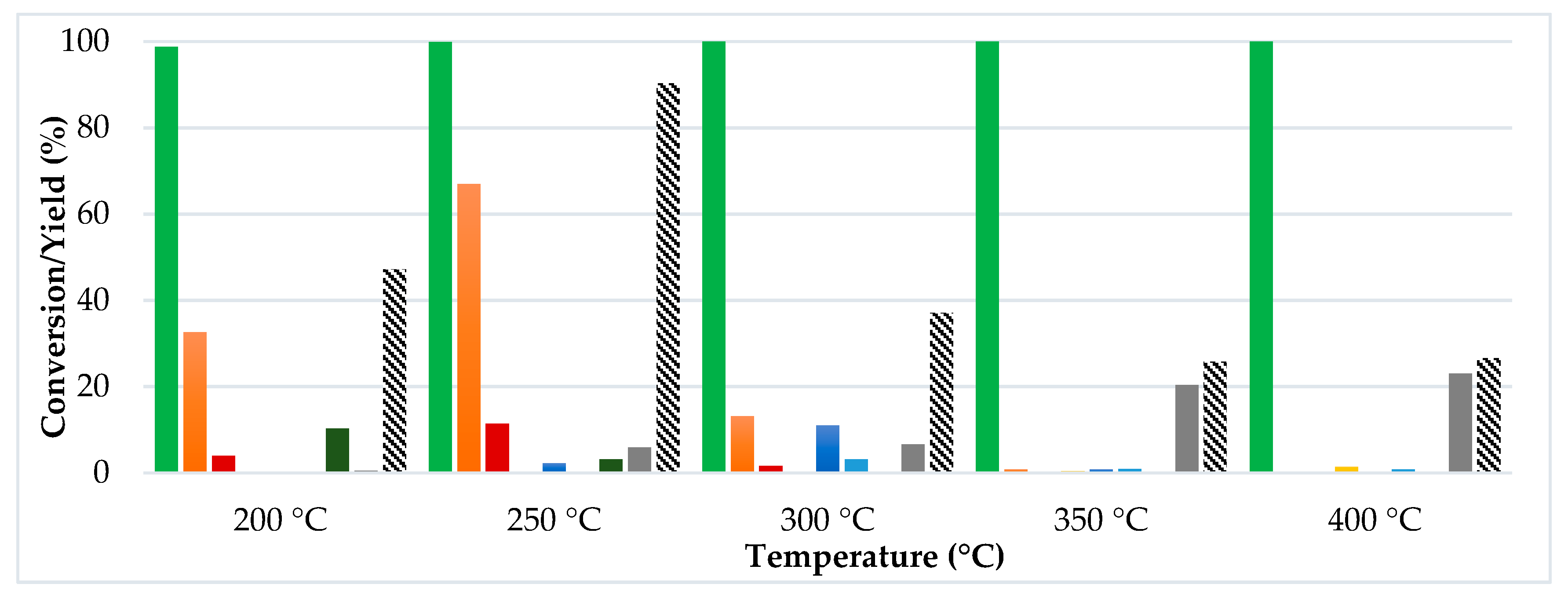
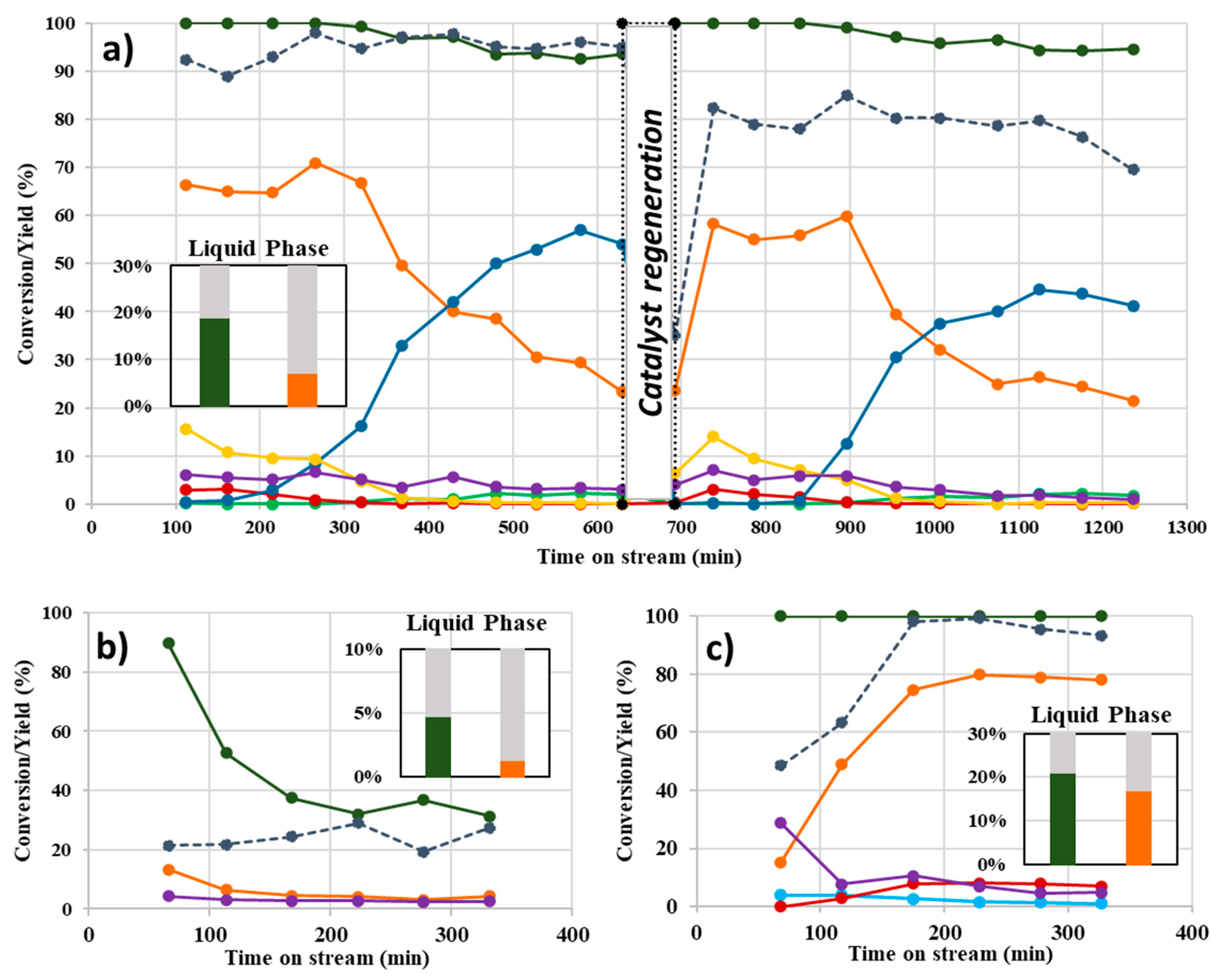
- The gas-phase continuous flow conditions produced a superior activity (higher ML conversion) and greater yield of GVL compared to the liquid-phase, regardless the alcohol used as the H-donor;
- Very poor results were obtained using methanol as the H-donor for the target reaction in terms of the desired yield of products. Further investigations using ethyl levulinate (EL) as the chosen substrate have proved the preferential tendency of methanol to promote both trans-esterification and alcoholysis of the intermediate (angelica lactones) reactions;
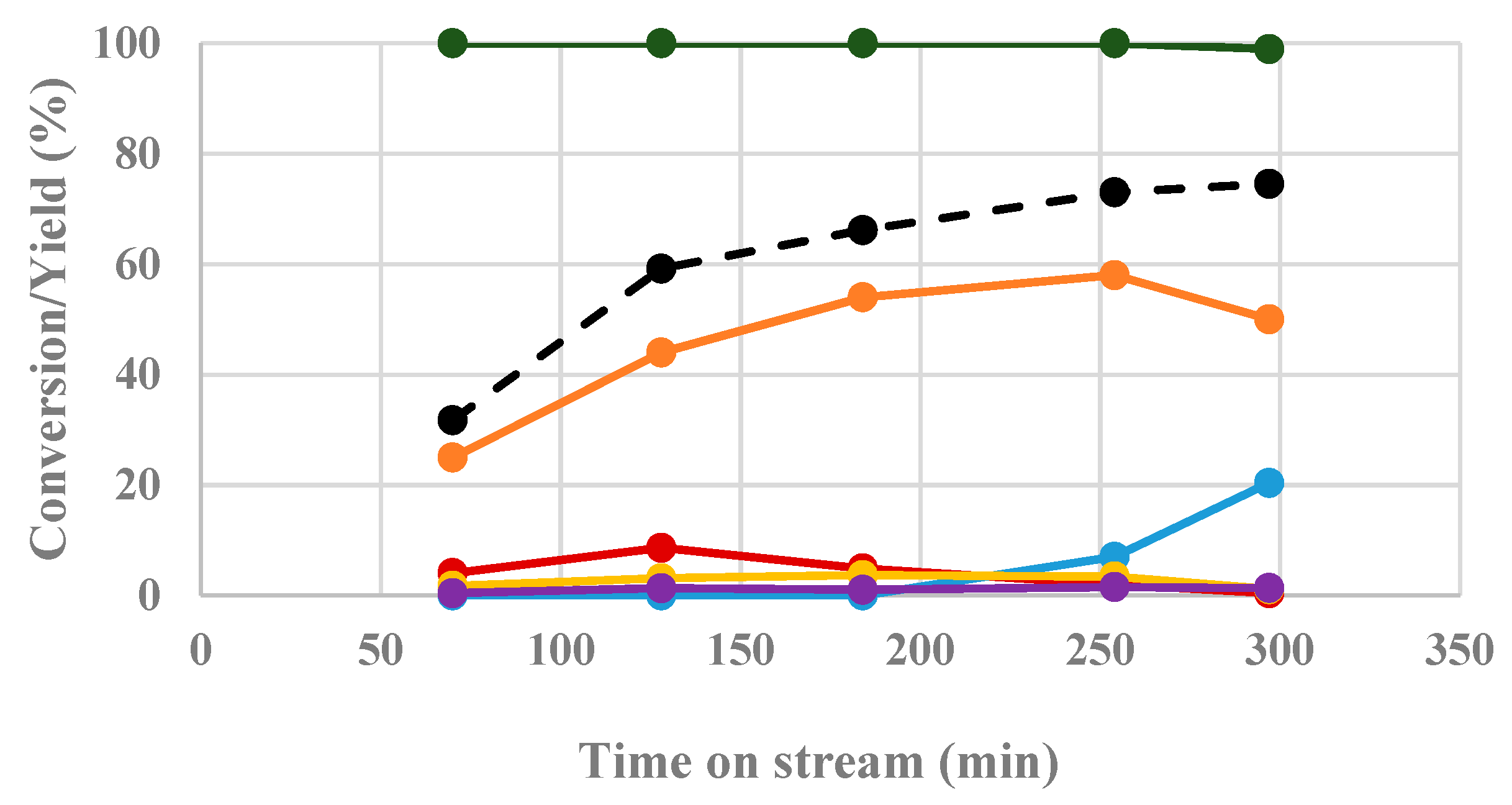
- Isopropanol has been confirmed as the best H-donor for the liquid-phase conditions while, in the continuous-flow system in the gas-phase conditions, ethanol and isopropanol led to very similar results with complete conversion of ML (at least for six hours of reaction) and a very good GVL yield (from 60 to 80%);
- When performing the catalytic tests using EL as the substrate (instead of ML), using the same reaction conditions as shown in Figure 2, a slight improvement in the obtained results in terms of catalytic activity and GVL yield were observed. This phenomenon may be attributed, to a limited extent, to the increased efficiency of EtOH as the leaving group in the intramolecular cyclization of EL to angelica lactones [26].
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ghosh, D.; Dasgupta, D.; Agrawal, D.; Kaul, S.; Adhikari, D.K.; Kurmi, A.K.; Arya, P.K.; Bangwal, D.; Negi, M.S. Fuels and Chemicals from Lignocellulosic Biomass: An Integrated Biorefinery Approach. Energy Fuels 2015, 29, 3149–3157. [Google Scholar] [CrossRef]
- Wettstein, S.G.; Martin Alonso, D.; Gürbüz, E.I.; Dumesic, J.A. Roadmap for Conversion of Lignocellulosic Biomass to Chemicals and Fuels. Curr. Opin. Chem. Eng. 2012, 1, 218–224. [Google Scholar] [CrossRef]
- Mukherjee, A.; Dumont, M.-J.; Raghavan, V. Review: Sustainable production of hydroxymethylfurfural and levulinic acid: Challenges and opportunities. Biomass Bioenergy 2015, 72, 143–183. [Google Scholar] [CrossRef]
- Rackemann, D.W.; Doherty, W.O. The conversion of lignocellulosics to levulinic acid. Biofuels Bioprod. Biorefining 2011, 5, 198–214. [Google Scholar] [CrossRef]
- Werpy, T.; Petersen, G. Top Value Added Chemicals from Biomass Volume I—Results of Screening for Potential Candidates from Sugars and Synthesis Gas; National Renewable Energy Lab.: Golden, CO, USA, 2004. [Google Scholar] [CrossRef]
- Omoruyi, U.; Page, S.; Hallett, J.; Miller, P.W. Homogeneous Catalyzed Reactions of Levulinic Acid: To γ-Valerolactone and Beyond. ChemSusChem 2016, 9, 2037–2047. [Google Scholar] [CrossRef]
- Gilkey, M.J.; Xu, B. Heterogeneous Catalytic Transfer Hydrogenation as an Effective Pathway in Biomass Upgrading. ACS Catal. 2016, 6, 1420–1436. [Google Scholar] [CrossRef]
- Horváth, I.T.; Mehdi, H.; Fábos, V.; Boda, L.; Mika, L.T. γ-Valerolactone a sustainable liquid for energy and carbon-based chemicals. Green Chem. 2008, 10, 238–242. [Google Scholar] [CrossRef]
- Upare, P.P.; Lee, J.M.; Hwang, D.W.; Halligudi, S.B.; Hwang, Y.K.; Chang, J.S. Selective Hydrogenation of Levulinic Acid to γ-Valerolactone over Carbon-Supported Noble Metal Catalysts. J. Ind. Eng. Chem. 2011, 17, 287–292. [Google Scholar] [CrossRef]
- Yan, Z.; Lin, L.; Liu, S. Synthesis of Gamma -Valerolactone by Hydrogenation of Biomass-Derived Levulinic Acid over Ru/C Catalyst. Energy Fuels 2009, 23, 3853–3858. [Google Scholar] [CrossRef]
- Sudhakar, M.; Kumar, V.V.; Naresh, G.; Lakshmi Kantam, M.; Bhargava, S.K. Vapor phase hydrogenation of aqueous levulinic acid over hydroxyapatite supported metal (M = Pd, Pt, Ru, Cu, Ni) catalysts. Appl. Catal. B Environ. 2016, 180, 113–120. [Google Scholar] [CrossRef]
- Boddien, A.; Loges, B.; Junge, H.; Beller, M. Hydrogen Generation at Ambient Conditions: Application in Fuel Cells. ChemSusChem 2008, 1, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Holladay, J.D.; Hu, J.; King, D.L.; Wang, Y. An Overview of Hydrogen Production Technologies. Catal. Today 2009, 139, 244–260. [Google Scholar] [CrossRef]
- Kuwahara, Y.; Kaburagi, W.; Osada, Y.; Fujitani, T.; Yamashita, H. Catalytic transfer hydrogenation of biomass-derived levulinic acid and its esters to gamma-valerolactone over ZrO2 catalyst supported on SBA-15 silica. Catal. Today 2017, 281, 418–428. [Google Scholar] [CrossRef]
- Scholz, D.; Aellig, C.; Hermans, I. Catalytic Transfer Hydrogenation/ Hydrogenolysis for Reductive Upgrading of Furfural and 5-(Hydroxymethyl)Furfural. ChemSusChem 2014, 7, 268–275. [Google Scholar] [CrossRef]
- Kuwahara, Y.; Kaburagi, W.; Fujitani, T. Catalytic Transfer Hydrogenation of Levulinate Esters to γ-Valerolactone over Supported Ruthenium Hydroxide Catalysts. RSC Adv. 2014, 4, 45848–45855. [Google Scholar] [CrossRef]
- Yang, Z.; Huang, Y.-B.; Guo, Q.-X.; Fu, Y. RANEY® Ni Catalyzed Transfer Hydrogenation of Levulinate Esters to γ- Valerolactone at Room Temperature. Chem. Commun. 2013, 49, 5328. [Google Scholar] [CrossRef]
- Komanoya, T.; Nakajima, K.; Kitano, M.; Hara, M. Synergistic Catalysis by Lewis Acid and Base Sites on ZrO2 for Meerwein-Ponndorf-Verley Reduction. J. Phys. Chem. C 2015, 119, 26540–26546. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, S.; Jaenicke, S.; Chuah, G. Zirconia catalysts in Meerwein-Ponndorf-Verley reduction of citral. Catal. Today 2004, 97, 249–255. [Google Scholar] [CrossRef]
- He, J.; Li, H.; Lu, Y.; Liu, Y.; Wu, Z.; Hu, D.; Yang, S. Cascade Catalytic Transfer Hydrogenation Cyclization of Ethyl Levulinate to Valerolactone with Al−Zr Mixed Oxides. Appl. Catal. A 2016, 510, 11–19. [Google Scholar] [CrossRef]
- Grazia, L.; Lolli, A.; Folco, F.; Zhang, Y.; Albonetti, S.; Cavani, F. Gas-Phase Cascade Upgrading of Furfural to 2-Methylfuran Using Methanol as a H-Transfer Reactant and MgO Based Catalysts. Catal. Sci. Technol. 2016, 6, 4418–4427. [Google Scholar] [CrossRef]
- Gliński, M.; Ulkowska, U. Reactivity of Alcohols in Chemoselective Transfer Hydrogenation of Acrolein over Magnesium Oxide as the Catalyst. Catal. Lett. 2011, 141, 293–299. [Google Scholar] [CrossRef]
- Chia, M.; Dumesic, J.A. Liquid-Phase Catalytic Transfer Hydrogenation and Cyclization of Levulinic Acid and Its Esters to γ-Valerolactone over Metal Oxide Catalysts. Chem. Commun. 2011, 47, 12233. [Google Scholar] [CrossRef] [PubMed]
- Neves, P.; Antunes, M.M.; Russo, P.A.; Abrantes, J.P.; Lima, S.; Fernandes, A.; Pillinger, M.; Rocha, S.M.; Ribeiro, M.F.; Valente, A.A. Production of Biomass-Derived Furanic Ethers and Levulinate Esters Using Heterogeneous Acid Catalysts. Green Chem. 2013, 15, 3367. [Google Scholar] [CrossRef]
- Tabanelli, T.; Paone, E.; Vásquez, P.B.; Pietropaolo, R.; Cavani, F.; Mauriello, F. Transfer Hydrogenation of Methyl and Ethyl Levulinate Promoted by a ZrO2 Catalyst: Comparison of Batch vs Continuous Gas-Flow Conditions. ACS Sustain. Chem. Eng. 2019, 7, 9937–9947. [Google Scholar] [CrossRef]
- Vásquez, P.B.; Tabanelli, T.; Monti, E.; Albonetti, S.; Bonincontro, D.; Dimitratos, N.; Cavani, F. Gas-Phase Catalytic Transfer Hydrogenation of Methyl Levulinate with Ethanol over ZrO2. ACS Sustain. Chem. Eng. 2019, 7, 8317–8330. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tabanelli, T.; Vásquez, P.B.; Paone, E.; Pietropaolo, R.; Dimitratos, N.; Cavani, F.; Mauriello, F. Improved Catalytic Transfer Hydrogenation of Levulinate Esters with Alcohols over ZrO2 Catalyst. Chem. Proc. 2020, 2, 28. https://doi.org/10.3390/ECCS2020-07585
Tabanelli T, Vásquez PB, Paone E, Pietropaolo R, Dimitratos N, Cavani F, Mauriello F. Improved Catalytic Transfer Hydrogenation of Levulinate Esters with Alcohols over ZrO2 Catalyst. Chemistry Proceedings. 2020; 2(1):28. https://doi.org/10.3390/ECCS2020-07585
Chicago/Turabian StyleTabanelli, Tommaso, Paola Blair Vásquez, Emilia Paone, Rosario Pietropaolo, Nikolaos Dimitratos, Fabrizio Cavani, and Francesco Mauriello. 2020. "Improved Catalytic Transfer Hydrogenation of Levulinate Esters with Alcohols over ZrO2 Catalyst" Chemistry Proceedings 2, no. 1: 28. https://doi.org/10.3390/ECCS2020-07585
APA StyleTabanelli, T., Vásquez, P. B., Paone, E., Pietropaolo, R., Dimitratos, N., Cavani, F., & Mauriello, F. (2020). Improved Catalytic Transfer Hydrogenation of Levulinate Esters with Alcohols over ZrO2 Catalyst. Chemistry Proceedings, 2(1), 28. https://doi.org/10.3390/ECCS2020-07585








