Influence of Carbonyl Position in C9 Ketones Against the Phytoparasitic Pinewood Nematode †
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Pinewood Nematode In Vitro Culture
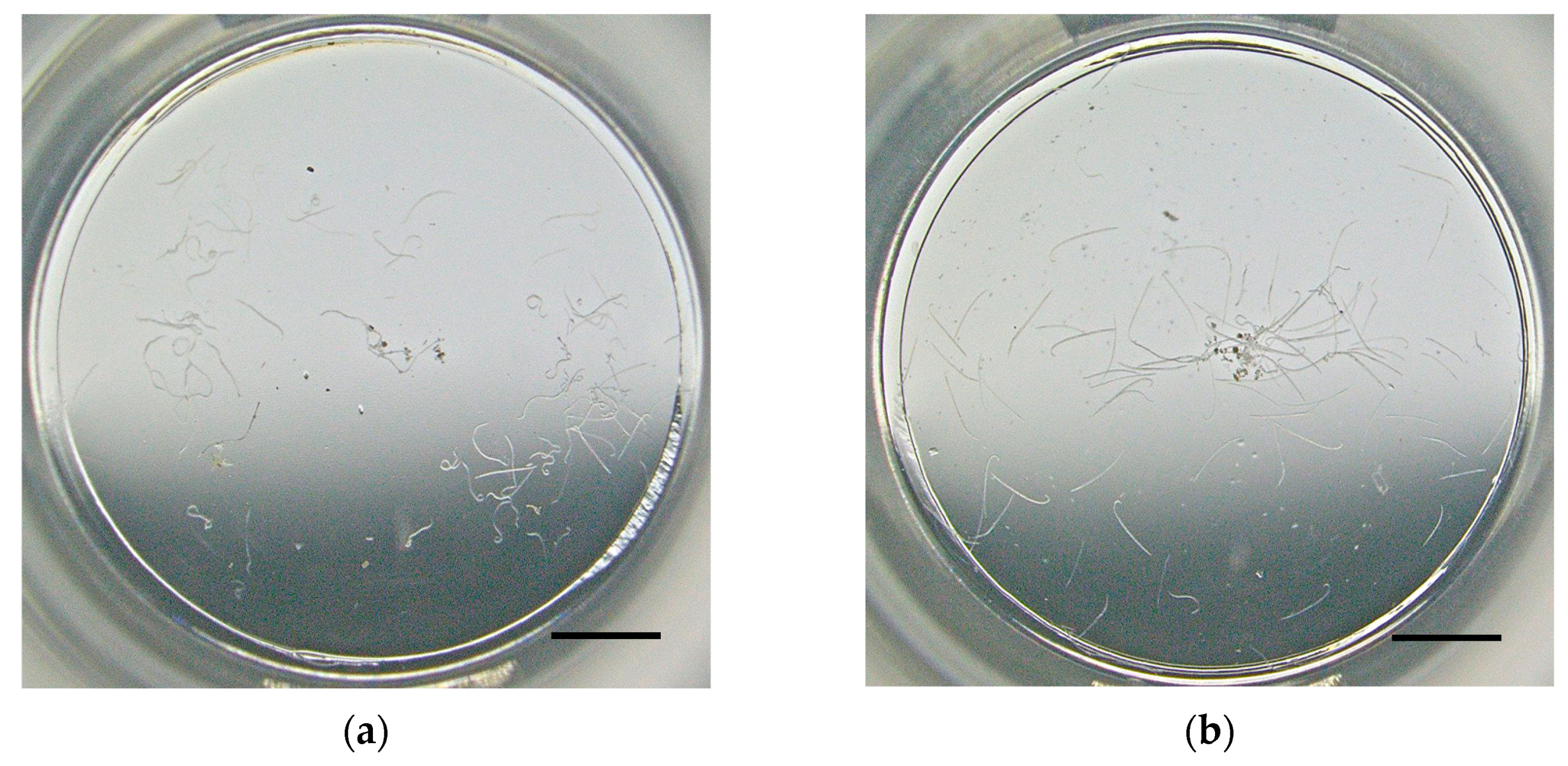
2.3. Direct-Contact Bioassays
2.4. Data Treatment and Statistical Analysis
3. Results and Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PWN | Pinewood Nematode |
| PWD | Pine Wilt Disease |
| SAR | Structure–Activity Relationship |
| HPLC | High-Performance Liquid Chromatography |
References
- Faria, J.M.S.; Sousa, E.; Bonifácio, L.; Carrasquinho, I.; Varela, A.R.; Inácio, M.L. Bursaphelenchus. In Compendium of Phytopathogenic Microbes in Agro-Ecology; Amaresan, N., Kumar, K., Eds.; Springer Nature: Cham, Switzerland, 2025; pp. 353–386. [Google Scholar]
- Back, M.A.; Bonifácio, L.; Inácio, M.L.; Mota, M.; Boa, E. Pine Wilt Disease: A Global Threat to Forestry. Plant Pathol. 2024, 73, 1026–1041. [Google Scholar] [CrossRef]
- Cavaco, T.; Faria, J.M.S. Phytochemical Volatiles as Potential Bionematicides with Safer Ecotoxicological Properties. Toxics 2024, 12, 406. [Google Scholar] [CrossRef] [PubMed]
- Sarri, K.; Mourouzidou, S.; Ntalli, N.; Monokrousos, N. Recent Advances and Developments in the Nematicidal Activity of Essential Oils and Their Components against Root-Knot Nematodes. Agronomy 2024, 14, 213. [Google Scholar] [CrossRef]
- European Parliament and Council. Regulation (ec) no 1107/2009; The European Parliament and of the Council of 21 october 2009 Concerning the Placing of Plant Protection Products on the Market and Repealing Council Directives 79/117/EEC and 91/414/EEC. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex:32009R1107 (accessed on 29 March 2025).
- Garrido, A.; Atencio, L.A.; Bethancourt, R.; Bethancourt, A.; Guzmán, H.; Gutiérrez, M.; Durant-Archibold, A.A. Antibacterial Activity of Volatile Organic Compounds Produced by the Octocoral-Associated Bacteria Bacillus Sp. BO53 and Pseudoalteromonas Sp. GA327. Antibiotics 2020, 9, 923. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.-M.; Kim, J.; Kim, E.; Park, H.-M.; Kim, Y.-J.; Park, I.-K. Structure−Activity Relationship of Aliphatic Compounds for Nematicidal Activity against Pine Wood Nematode (Bursaphelenchus Xylophilus). J. Agric. Food Chem. 2010, 58, 1823–1827. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.M.; Kim, J.; Koh, S.H.; Ahn, Y.J.; Park, I.K. Nematicidal Activity of Natural Ester Compounds and Their Analogues against Pine Wood Nematode, Bursaphelenchus Xylophilus. J. Agric. Food Chem. 2014, 62, 9103–9108. [Google Scholar] [CrossRef] [PubMed]
- Faria, J.M.S.; Barbosa, P. Cymbopogon Citratus Allelochemical Volatiles as Potential Biopesticides against the Pinewood Nematode. Plants 2024, 13, 2233. [Google Scholar] [CrossRef] [PubMed]
- Faria, J.M.S.; Cavaco, T.; Gonçalves, D.; Barbosa, P.; Teixeira, D.M.; Moiteiro, C.; Inácio, M.L. First Report on the Synergistic Interaction between Essential Oils against the Pinewood Nematode Bursaphelenchus Xylophilus. Plants 2023, 12, 2438. [Google Scholar] [CrossRef] [PubMed]
- Faria, J.M.S.; Figueiredo, A.C.; Teixeira, D.M.; Inácio, M.L. Infection of In Vivo and In Vitro Pines with the Pinewood Nematode Bursaphelenchus Xylophilus and Isolation of Induced Volatiles. J. Vis. Exp. 2024, 211, e67149. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, A.G.; Hemming, J.R. A Comparison of Some Quantitative Methods of Extracting Small Vermiform Nematodes from Soil. Ann. Appl. Biol. 1965, 55, 25–38. [Google Scholar] [CrossRef]
- Faria, J.M.S.; Pereira, G.; Figueiredo, A.C.; Barbosa, P. In Vivo and In Vitro Grown Lemon-Scented Gum as a Source of Nematicidal Essential Oil Compounds. Plants 2025, 14, 1892. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.O.; Lee, S.M.; Moon, Y.S.; Lee, S.G.; Ahn, Y.J. Nematicidal Activity of Plant Essential Oils against Bursaphelenchus Xylophilus (Nematoda: Aphelenchoididae). J. Asia. Pac. Entomol. 2006, 9, 173–178. [Google Scholar] [CrossRef]
- Kim, J.; Lee, S.J.; Park, J.O.; Yoon, K.A. Nematicidal Activity of Benzyloxyalkanols against Pine Wood Nematode (Bursaphelenchus xylophilus). Biomolecules 2021, 11, 384. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-R.; Lee, S.-C.; Lee, J.-E.; Seo, S.-M.; Jeong, Y.-C.; Jung, C.-S.; Moloney, M.G.; Park, I.-K. Nematicidal Activity of 3-Acyltetramic Acid Analogues against Pine Wood Nematode, Bursaphelenchus xylophilus. Molecules 2017, 22, 1568. [Google Scholar] [CrossRef] [PubMed]
| Ketones | Chemical Structure | PWN Mortality 1 | Nematicidal Strength |
|---|---|---|---|
| 2-Nonanone | 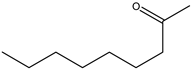 | 92.3 ± 1.2 | Strong |
| 3-Nonanone |  | 80.1 ± 0.8 | Strong |
| 5-Nonanone |  | 17.1 ± 0.5 | Low/inactive |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faria, J.M.S.; Pereira, G. Influence of Carbonyl Position in C9 Ketones Against the Phytoparasitic Pinewood Nematode. Chem. Proc. 2025, 18, 61. https://doi.org/10.3390/ecsoc-29-26710
Faria JMS, Pereira G. Influence of Carbonyl Position in C9 Ketones Against the Phytoparasitic Pinewood Nematode. Chemistry Proceedings. 2025; 18(1):61. https://doi.org/10.3390/ecsoc-29-26710
Chicago/Turabian StyleFaria, Jorge M. S., and Gonçalo Pereira. 2025. "Influence of Carbonyl Position in C9 Ketones Against the Phytoparasitic Pinewood Nematode" Chemistry Proceedings 18, no. 1: 61. https://doi.org/10.3390/ecsoc-29-26710
APA StyleFaria, J. M. S., & Pereira, G. (2025). Influence of Carbonyl Position in C9 Ketones Against the Phytoparasitic Pinewood Nematode. Chemistry Proceedings, 18(1), 61. https://doi.org/10.3390/ecsoc-29-26710







