Understanding the Therapeutic Potential of Quercetin and Resveratrol: Computational Insights into Antidiabetic Activity †
Abstract
1. Introduction
2. Materials and Methods
2.1. Target Prediction
2.2. Structure Preparation of Ligands and Proteins
2.3. Prediction of Protein–Ligand Interactions
3. Results and Discussion
3.1. Analysis of Target Predictions
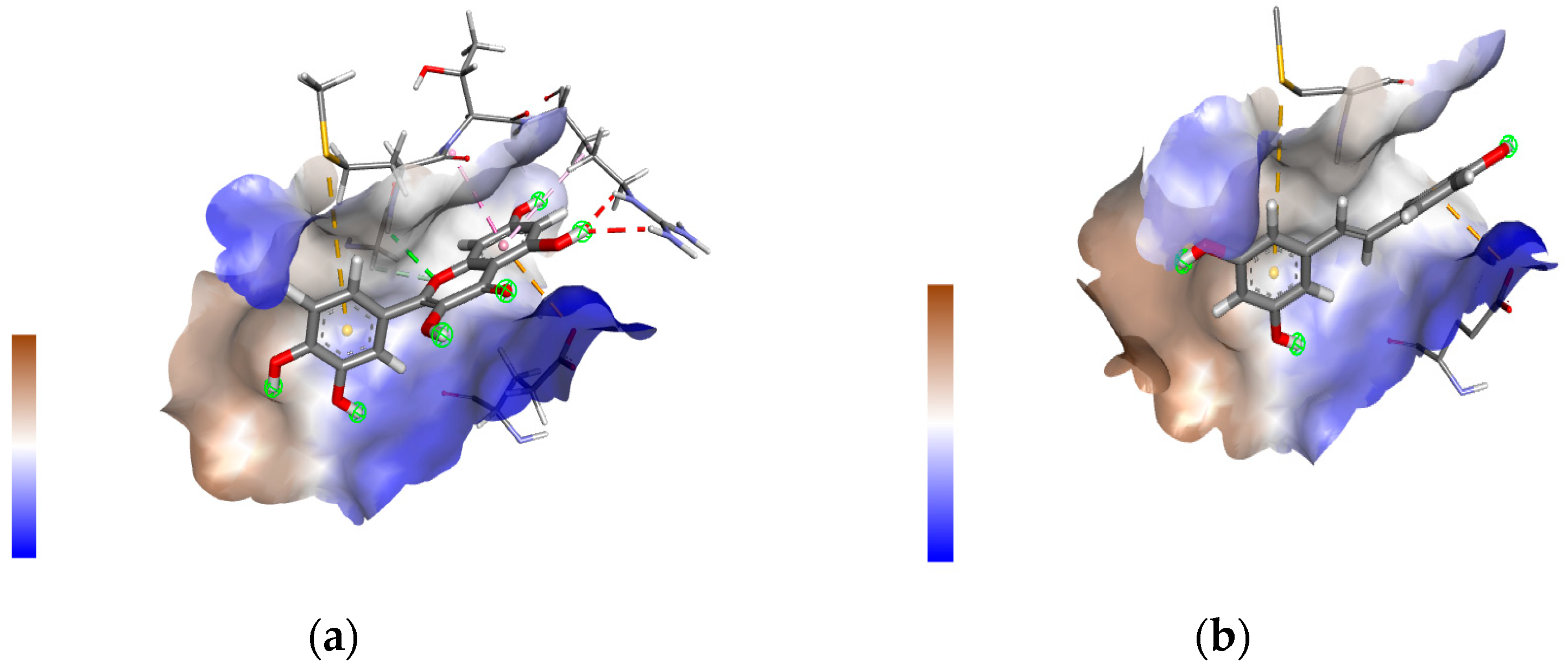
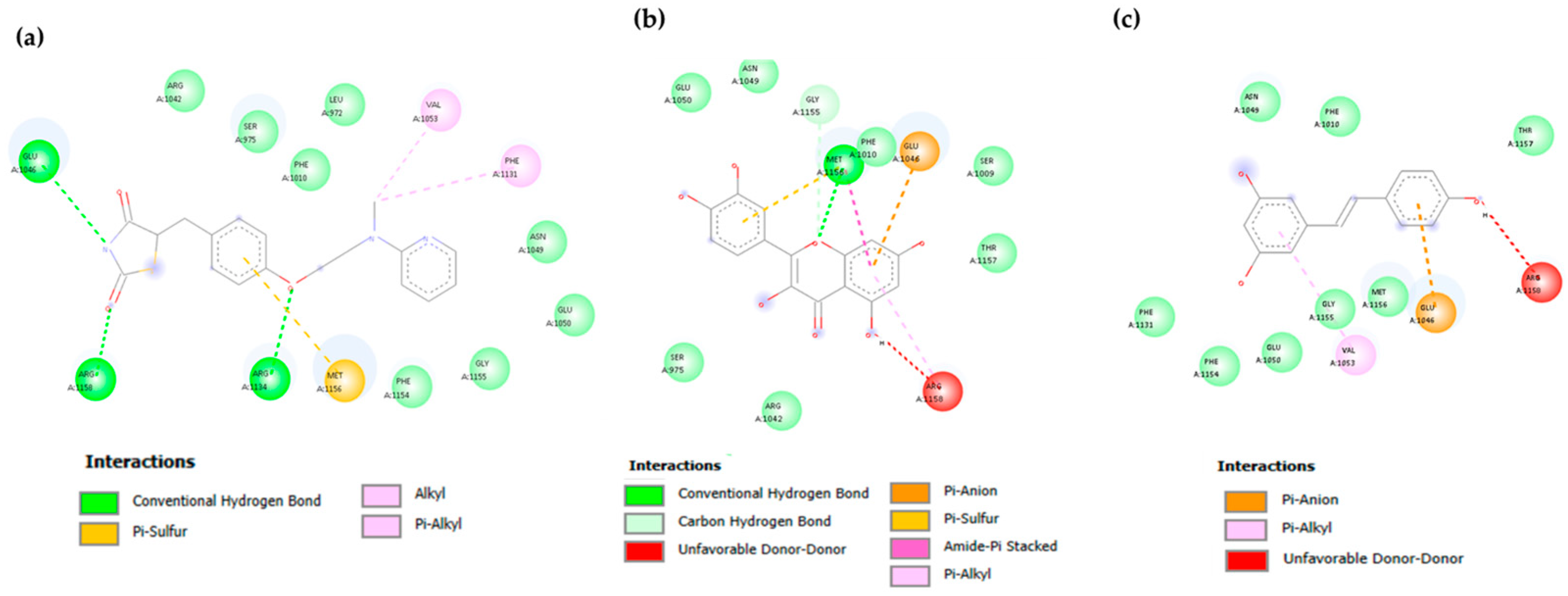
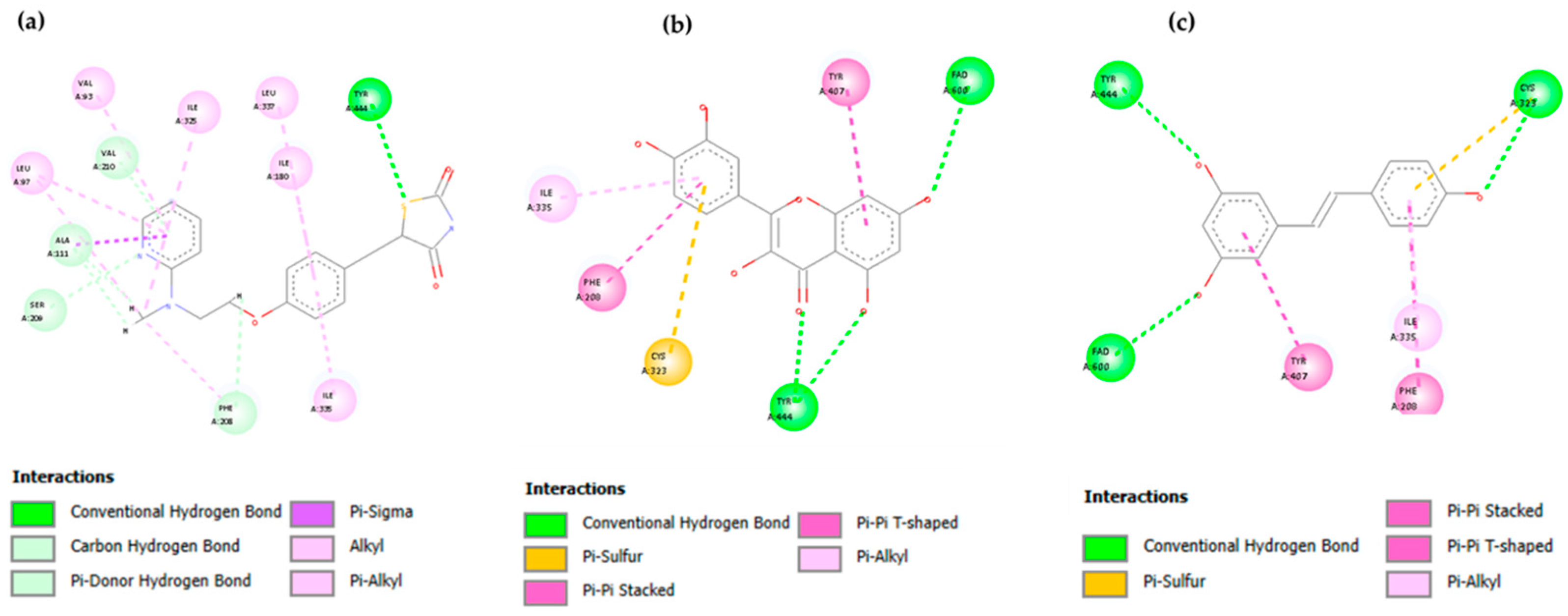
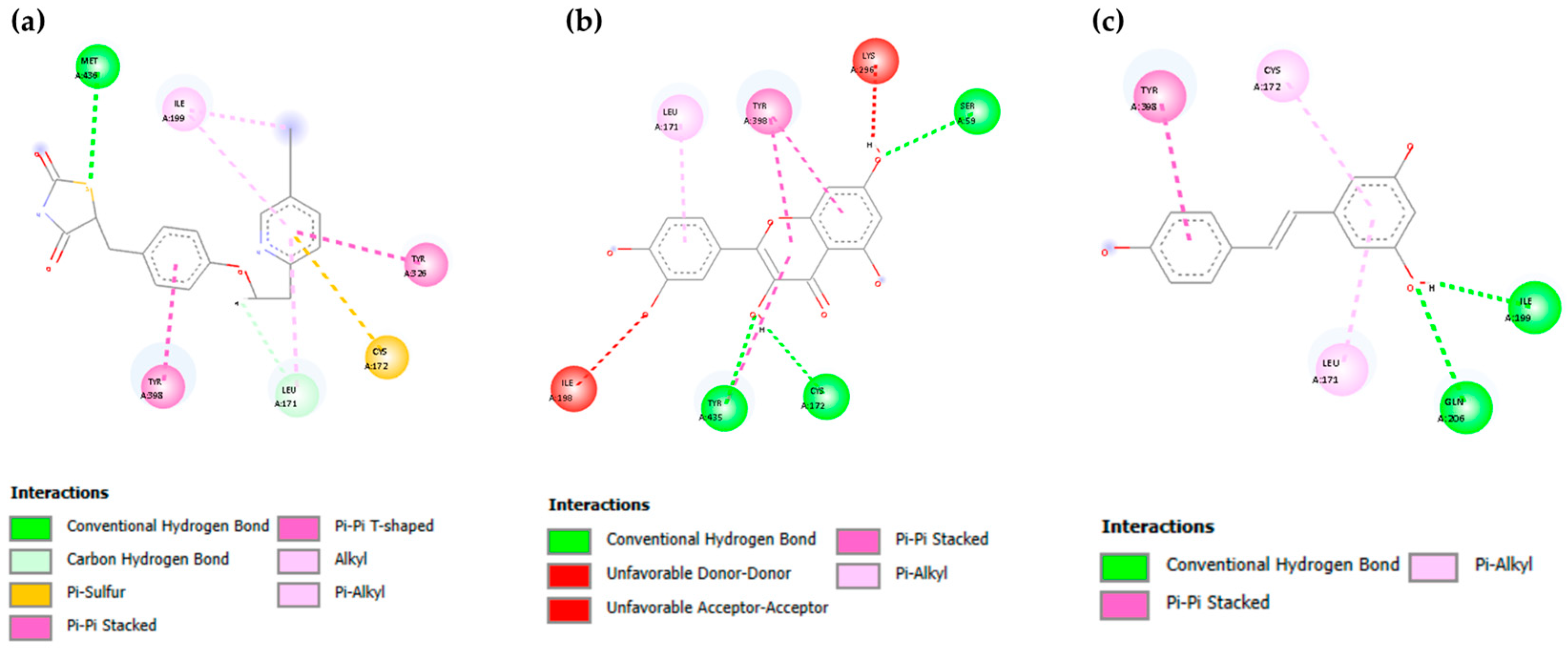
3.2. Molecular Docking
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Niccoli, T.; Partridge, L. Ageing as a Risk Factor for Disease. Curr. Biol. 2012, 22, R741–R752. [Google Scholar] [CrossRef] [PubMed]
- Deepika; Maurya, P.K. Health Benefits of Quercetin in Age-Related Diseases. Molecules 2022, 27, 2498. [Google Scholar] [CrossRef] [PubMed]
- Mantadaki, A.E.; Linardakis, M.; Tsakiri, M.; Baliou, S.; Fragkiadaki, P.; Vakonaki, E.; Tzatzarakis, M.N.; Tsatsakis, A.; Symvoulakis, E.K. Benefits of Quercetin on Glycated Hemoglobin, Blood Pressure, PiKo-6 Readings, Night-Time Sleep, Anxiety, and Quality of Life in Patients with Type 2 Diabetes Mellitus: A Randomized Controlled Trial. J. Clin. Med. 2024, 13, 3504. [Google Scholar] [CrossRef] [PubMed]
- Ahmadzadeh, A.M.; Aliabadi, M.M.; Mirheidari, S.B.; Hamedi-Asil, M.; Garousi, S.; Mottahedi, M.; Sahebkar, A. Beneficial effects of resveratrol on diabetes mellitus and its complications: Focus on mechanisms of action. Naunyn Schmiedebergs Arch. Pharmacol. 2025, 398, 2407–2442. [Google Scholar] [CrossRef] [PubMed]
- Basagni, F.; Di Paolo, M.L.; Cozza, G.; Dalla Via, L.; Fagiani, F.; Lanni, C.; Rosini, M.; Minarini, A. Double Attack to Oxidative Stress in Neurodegenerative Disorders: MAO-B and Nrf2 as Elected Targets. Molecules 2023, 28, 7424. [Google Scholar] [CrossRef] [PubMed]
- Binda, C.; Aldeco, M.; Geldenhuys, W.J.; Tortorici, M.; Mattevi, A.; Edmondson, D.E. Molecular Insights into Human Monoamine Oxidase B Inhibition by the Glitazone Antidiabetes Drugs. ACS Med. Chem. Lett. 2012, 3, 39–42. [Google Scholar] [CrossRef] [PubMed]
- Almeida-Nunes, D.L.; Silva, J.P.N.; Nunes, M.; Silva, P.M.A.; Silvestre, R.; Dinis-Oliveira, R.J.; Bousbaa, H.; Ricardo, S. Metformin Impairs Linsitinib Anti-Tumor Effect on Ovarian Cancer Cell Lines. Int. J. Mol. Sci. 2024, 25, 11935. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Liu, Y.; Zhu, F.; Liu, S.; Cai, Z.; Liu, M.; An, X.; Yao, Y.; Chen, N.; Guo, D. Picropodophyllin induces ferroptosis via blockage of AKT/NRF2/SLC7A11 and AKT/NRF2/SLC40A1 axes in hepatocellular carcinoma as a natural IGF1R inhibitor. Phytomedicine 2025, 143, 156840. [Google Scholar] [CrossRef] [PubMed]
- Gfeller, D.; Grosdidier, A.; Wirth, M.; Daina, A.; Michielin, O.; Zoete, V. SwissTargetPrediction: A web server for target prediction of bioactive small molecules. Nucleic Acids Res. 2014, 42, W32–W38. [Google Scholar] [CrossRef] [PubMed]
- Oladipo, S.D.; Luckay, R.C.; Olalekan, S.O.; Badeji, A.A.; Matinise, N.; Tshikhudo, F. Investigating the Inhibitory Potential of Halogenated Quinoline Derivatives against MAO-A and MAO-B: Synthesis, Crystal Structure, Density Functional Theory, and Molecular Dynamics Simulations. ACS Omega 2025, 10, 26500–26519. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Pang, L.; Wang, H.; Han, Q.; Wan, W.; Luo, S.; Song, Z.; Fang, Y.; Chen, H.; Qiu, Y.; et al. Comprehensive Analysis of Uric Acid and Myasthenia Gravis: IGF1R as a Protective Factor and Potential Therapeutic Target. CNS Neurosci. Ther. 2025, 31, e70361. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, S.; Ajadee, A.; Sarker, A.; Ahmmed, R.; Noor, T.; Pappu, M.A.A.; Islam, M.S.; Mollah, M.N.H. Exploring common genomic biomarkers to disclose common drugs for the treatment of colorectal cancer and hepatocellular carcinoma with type-2 diabetes through transcriptomics analysis. PLoS ONE 2025, 20, e0319028. [Google Scholar]
- Carroll, R.T.; Dluzen, D.E.; Stinnett, H.; Awale, P.S.; Funk, M.O.; Geldenhuys, W.J. Structure–activity relationship and docking studies of thiazolidinedione-type compounds with monoamine oxidase B. Bioorg. Med. Chem. Lett. 2011, 21, 4798–4803. [Google Scholar] [PubMed]
| Target Name | Common Name | Probability Quercetin | Probability Resveratrol |
|---|---|---|---|
| NADPH oxidase 4 | NOX4 | 1.0 | 0 |
| Monoamine oxidase A | MAO -A | 1.0 | 1.0 |
| Insulin-like growth factor | IGF-1R | 1.0 | 0.049 |
| Cytochrome P450 19A1 | CYP19A1 | 1.0 | 0.058 |
| Epidermal growth factor | EGFR | 1.0 | 0.049 |
| Tyrosine-protein kinase receptor | FLT3 | 1.0 | 0 |
| Arachidonate 5-lipoxygenase | ALOX5 | 1.0 | 0.058 |
| Molecule Name | Therapeutical Indication | MAO-A | MAO-B | IGF1R |
|---|---|---|---|---|
| Linsitinib | anticancer | −8.1 | −9.8 | −5.9 |
| Metformin | antidiabetic | −5.6 | −4.8 | −4.2 |
| Picropodophyllin | anticancer | 6.5 | −9.2 | −7.5 |
| Pioglitazone | antidiabetic | −8.2 | −10.2 | −6.8 |
| Rosiglitazone | antidiabetic | −8.1 | −9.7 | −6.9 |
| Quercetin | antioxidant | −7.8 | −9.4 | −6.9 |
| Resveratrol | antioxidant | −8.4 | −8.5 | −6.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Markočević, M.; Ivković, M.; Zloh, M. Understanding the Therapeutic Potential of Quercetin and Resveratrol: Computational Insights into Antidiabetic Activity. Chem. Proc. 2025, 18, 53. https://doi.org/10.3390/ecsoc-29-26876
Markočević M, Ivković M, Zloh M. Understanding the Therapeutic Potential of Quercetin and Resveratrol: Computational Insights into Antidiabetic Activity. Chemistry Proceedings. 2025; 18(1):53. https://doi.org/10.3390/ecsoc-29-26876
Chicago/Turabian StyleMarkočević, Mirna, Milena Ivković, and Mire Zloh. 2025. "Understanding the Therapeutic Potential of Quercetin and Resveratrol: Computational Insights into Antidiabetic Activity" Chemistry Proceedings 18, no. 1: 53. https://doi.org/10.3390/ecsoc-29-26876
APA StyleMarkočević, M., Ivković, M., & Zloh, M. (2025). Understanding the Therapeutic Potential of Quercetin and Resveratrol: Computational Insights into Antidiabetic Activity. Chemistry Proceedings, 18(1), 53. https://doi.org/10.3390/ecsoc-29-26876







