1. Introduction
The aldol reaction is a key transformation that enables the synthesis of β-hydroxy ketones through the reaction between a ketone and an aldehyde, typically in the presence of a secondary amine acting as a catalyst. One of the most representative examples of this type of catalysis is that mediated by the amino acid proline [
1,
2,
3]. This small molecule efficiently promotes carbon–carbon bond formation in aldol reactions via the formation of an iminium or enamine intermediate with the carbonyl substrate [
4]. Currently, the significance of this amino acid is such that the work reported by List et al. [
5]. demonstrating its catalytic ability to generate high enantiomeric excesses has been foundational. As a result, asymmetric catalysis has become one of the cornerstones in the synthesis of high-value molecules, such as pharmaceuticals. Moreover, List’s pioneering work was recognized with the Nobel Prize in Chemistry in 2021 [
6,
7]. Inspired by proline, a new generation of proline-derived organocatalysts such as prolinamides and other peptides has demonstrated excellent catalytic performance [
8,
9,
10]. In addition, innovative approaches to the aldol reaction have emerged, including solvent-free conditions [
10], mechanochemical activation techniques [
11], and the use of bifunctional catalysts. In the latter, transition metal complexes are often employed, with a preference for metals such as zinc [
12], cobalt [
13], zirconium [
14,
15] or palladium. This latter metal has gained significant attention due to its ability to form bifunctional catalysts that incorporate both proline and palladium (Pd) within a metal–organic framework (MOF), thereby enabling one-pot C–C bond-forming reactions such as Suzuki/aldol cascade [
15]. Additionally, trifunctional catalytic systems have been developed, comprising cobalt nanoparticles, Pd(II), and proline supported on UiO-67 to form MOFs that efficiently promote oxidation/Heck/aldol sequential reactions [
16].
2. Results and Discussion
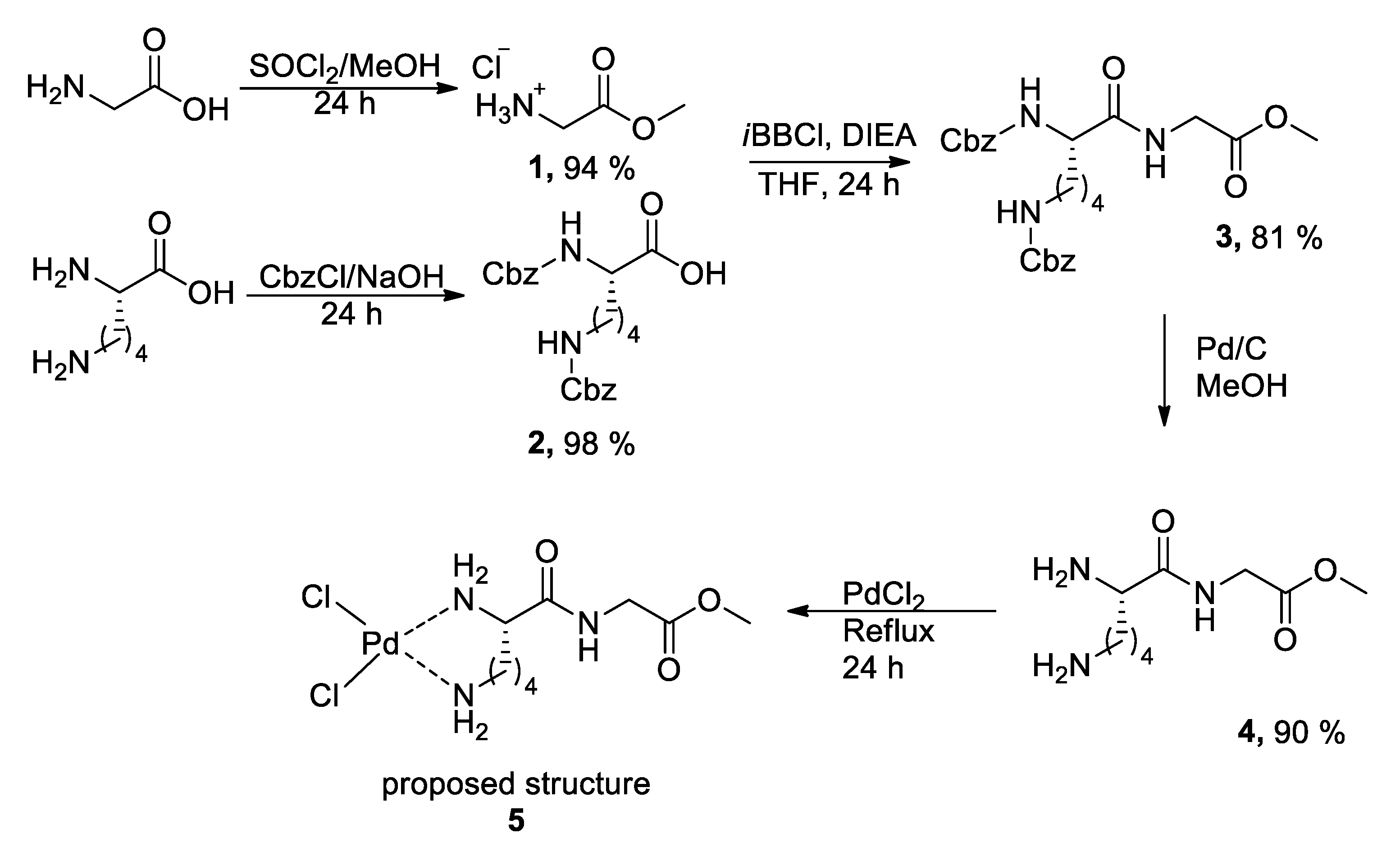
In the present work, the synthesis of a Pd(II) coordination complex with the peptide
L-Lys-Gly as a ligand is described, along with the investigation of its ability to promote the stereoselective formation of C–C bonds through aldol and Heck reactions. The synthetic approach began with the esterification of glycine (Gly) and the dual protection of the α- and ε-amino groups of
L-lysine (Lys). Following a liquid-phase synthesis protocol via mixed anhydrides [
17], the peptide bond between the two amino acids was formed through amide coupling, affording the fully protected dipeptide
3 in good yield (
Scheme 1). Finally, the Cbz protecting group was removed by catalytic hydrogenation of compound
3 using 10% Pd/C in methanol, deprotecting the amino functionalities and yielding the
L-Lys-Gly ester
4 in good overall yield.
Subsequently, the reaction of dipeptide
4 with PdCl
2 was carried out. After 24 h of heating, a noticeable color change was observed. The reaction yielded a black solid with a melting point of 216–218 °C (notably higher than that of compound
3, 180–182 °C). This transformation was further supported by scanning electron microscopy (SEM) coupled with energy-dispersive X-ray spectroscopy (EDS), which confirmed the presence of palladium coordinated to the organic molecule. The EDS spectrum showed the characteristic Kα emission peak for palladium at 3.382 keV, as well as the chlorine signal at 2.622 keV (
Figure 1a).
The infrared spectrum (IR) indicated that the stretching frequencies of most functional groups remained largely unchanged. Specifically, the C=O stretching band was maintained at 1671 cm
−1, and similar behavior was observed for the symmetric and asymmetric C–H stretching bands. In contrast, significant changes were observed in the bands associated with –NH
2 groups. In the spectrum of the free ligand (compound
4,
Figure 1b, red FTIR trace), the symmetric and asymmetric N–H stretching bands appeared at 3349 cm
−1 and 3202 cm
−1, respectively. In the spectrum of complex
5 (
Figure 1b, blue FTIR trace), a broadening of the N–H band at 3202 cm
−1 was observed, along with a weakening of the symmetric N–H band at 3349 cm
−1. Additionally, a band at 1398 cm
−1 corresponding to C–N stretching was identified.
This behavior is attributed to the formation of a Pd–N coordination bond, which weakens the N–H bond, resulting in a shift in the corresponding vibrational frequency to lower values, an effect also observed in Pt(II) complexes with amines [
18]. Additionally, the NMR spectrum of complex
5, compared to that of the free ligand (
Figure S1), shows signal broadening and significant shifts in chemical shifts, particularly in the methylene protons of the lysine side chain. These results support the hypothesis that the complex is formed through bidentate chelation involving both the α-NH
2 and ε-NH
2 groups of the lysine residue. This coordination mode is consistent with previous reports describing the formation of metallacycles of similar or larger ring sizes [
18,
19].
On the other hand, the formation of a monodentate complex is considered energetically unfavorable due to the length of the lysine side chain. It is also worth noting that the pKa values of both amino groups are very similar. Moreover, since both donor groups are located within the same organic fragment, the formation of a bidentate N–Pd–N chelate complex is favored.
As a subsequent step, functional groups present in the triprotected dipeptide that do not participate in coordination bond formation were excluded. To this end, the triprotected dipeptide (compound
3) was reacted with 2.1 equivalents of PdCl
2 [
20]. After 24 h of reflux, only the starting material was recovered, as confirmed by
1H NMR and FTIR spectroscopy (
Figures S2 and S3). This result confirms the stability of the ester group and provides evidence that the oxygen lone pairs of the carboxyl group or the lone pairs on the nitrogen atoms of the carbamate or amide groups do not participate in coordination bond formation (see
Supporting Information).
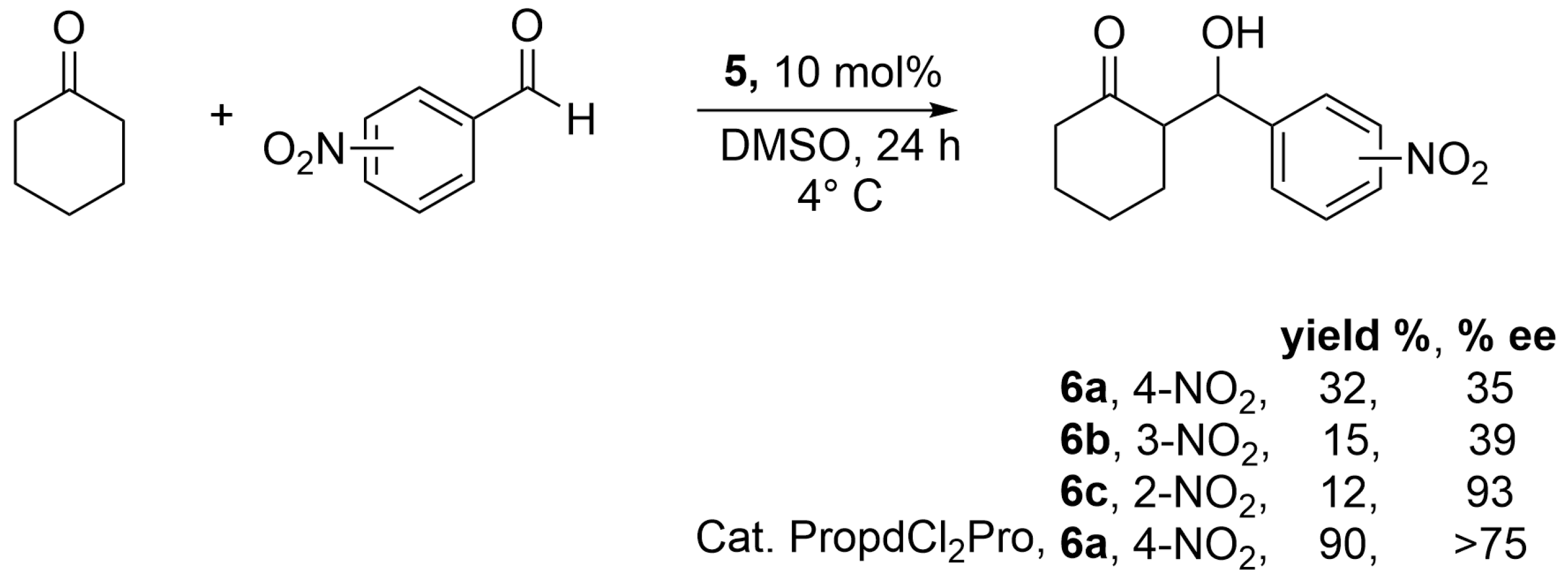
Once complex
5 was identified, its catalytic ability to promote C–C bond formation and induce chirality was evaluated via the aldol reaction between cyclohexanone and 4-nitrobenzaldehyde at room temperature, aiming to obtain adduct
6a (
Scheme 2).
In an initial screening, various solvents were tested: H2O, EtOH, DMF, DMSO, CH3CN, and THF. From this preliminary analysis, the reaction did not proceed in H2O, THF, or CH3CN. Conversely, the reaction occurred in EtOH, DMF, and DMSO, albeit with modest yields. Regarding diastereoselectivity, syn/anti ratios of 1:1 were obtained in all cases. The adducts exhibited enantiomeric excesses of 16% in EtOH and 24% and 27% in DMF and DMSO, respectively. These results highlight the importance of the solvent in catalyst solvation and, consequently, in the induction of chirality.
Subsequently, performing the reaction at 4 °C resulted in an increase in enantioselectivity, reaching a value of 35% ee. Once these optimal conditions were established, the reaction was evaluated using 2-, 3-, and 4-nitrobenzaldehyde for the synthesis of hydroxyketones
6a–
c (
Scheme 3).
The results showed no diastereoselectivity in all cases; however, enantioselectivities exceeded 50% ee when the –NO2 substituent was located at the 2-position. It is noteworthy that enantioselectivity values decreased as the deactivating group was positioned further from the aldehyde carbonyl, with 39% ee for 6b and 32% ee for 6a.
Additionally, it was confirmed that the size of the catalyst does not directly influence stereoinduction. To support this hypothesis, the aldol reaction was performed using the coordination complex previously reported by Aviña-Verduzco et al. [
20] which contains proline as the ligand. Under the same reaction conditions, this catalyst achieved good yields and enantiomeric excesses greater than 75%.
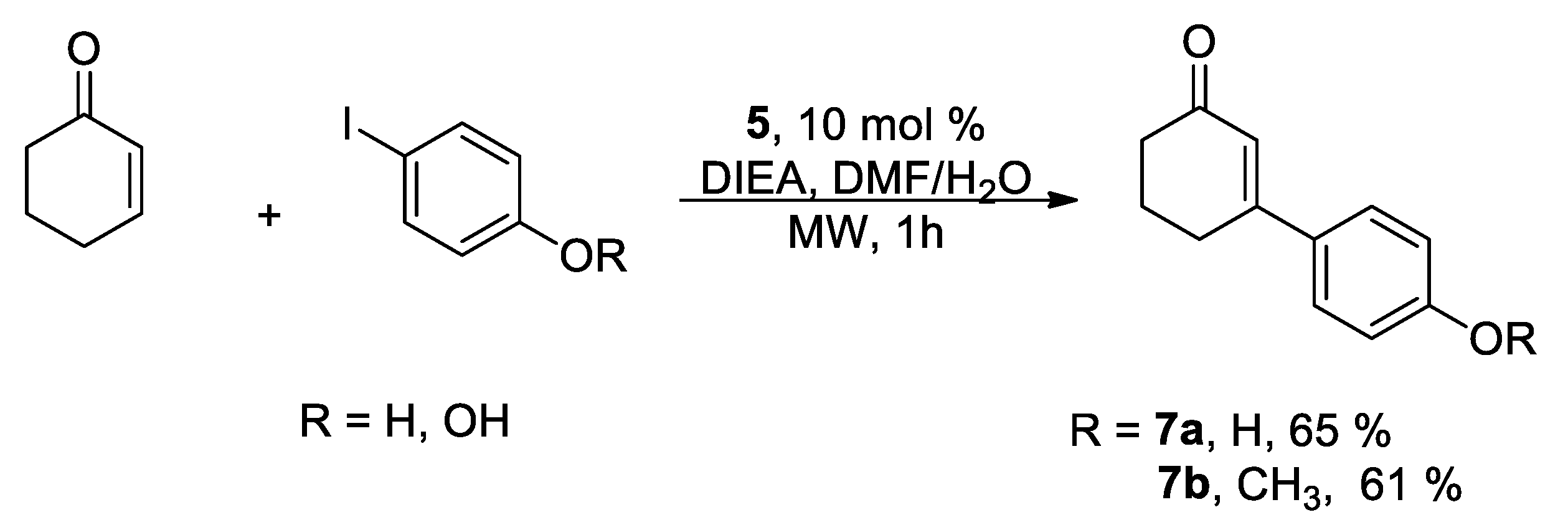
To further evaluate the scope of catalyst
5, the Heck cross-coupling reaction was carried out between aryl halides 4-iodophenol and 4-iodoanisole with 2-cyclohexen-1-one (
Scheme 4). After 60 min of reaction time, good yields were observed with both halides. It is noteworthy that, due to the presence of water in the reaction medium, partial precipitation of the catalyst occurs upon completion of the reaction, enabling its potential recovery and reuse.
3. Experimental Section
3.1. General
Column chromatography was carried out with Merck Sigma-Aldrich (Milwaukee, WI, USA). Silica Gel (70–230 mesh). TLC was performed with Merck 60-F254 plates (Darmstadt, Germany), and spots were visualized using UV light and iodine vapor. Melting points were measured with a Fisher-Johns melting points apparatus model 5007 (Sunnyvale, CA, USA) and were left uncorrected. NMR spectra were recorded on a Varian Mercury plus (100 and 400 MHz) Palo Alto, CA, USA.
3.2. Procedure for Complex 5
In a flask equipped with a magnetic stirrer, a condenser, and a nitrogen atmosphere, 0.0224 g of PdCl2 was dissolved in HPLC-grade acetonitrile. The resulting reaction mixture was refluxed for 2 h, after which 0.0588 mmol of L-Lys-Gly-OMe was added and the reaction was allowed to continue under reflux for 18 h. Upon completion, a fine black solid was obtained (0.0421 g, 55% yield) with a melting point of 216–218 °C.
1H NMR (400 MHz, DMSOd6) δ: 3.73 (m, 1H, CH); 3.71 (dd, J = 19.7, 17.4, 2.4 Hz, 2H, CH2); 3.46 (t, J = 6.2 Hz, 2H, NH); 3.40 (s, 3H, CH3); 2.38 (dt, J = 14.1, 7.1 Hz, 2H, CH2); 1.62 (m, 2H, CH2); 1.62 (m, 2H, CH2); 1.30 (m, 2H, CH2), 13C NMR (100 MHz, DMSOd6) δ: 168.0, 166.2, 54.0, 54.0, 44.3, 43.9, 32.5, 30.1, 21.4.
3.3. General Procedure for the Aldol Reaction
In a flask equipped with magnetic stirring, 1.0 equiv of the corresponding aldehyde and 5.0 equiv of cyclohexanone were dissolved in 0.3 mL of DMSO. To the resulting solution, 10 mol% of the catalyst was added, and the mixture was stirred at 4 °C for 24 h. After this time, the reaction was quenched with a saturated aqueous solution of ammonium chloride. The mixture was extracted with EtOAc (3 × 25 mL) and distilled water (20 mL). The combined organic layers were dried over Na2SO4, filtered by gravity, and concentrated under reduced pressure.
3.3.1. 2-(Hydroxy(4-nitrophenyl)methyl)cyclohexanone (6a)
Following the general procedure for the aldol reaction, 4-nitrobenzaldehyde (30 mg, 1.19 mmol) and cyclohexanone (0.13 mL, 1.06 mmol) were placed in 0.3 mL of DMSO. The catalyst (7.8 mg, 0.01 mmol) was then added, and the reaction was stirred under the established conditions. After workup, the product was obtained (0.15 mg, yield 32%) as a white solid.
1H NMR (400 MHz, CDCl3) δ: 8.21 (d, J = 8.7 Hz, 2H), 7.51 (d, J = 8.6 Hz, 2H), 4.90 (d, J = 8.4 Hz, 1H), 4.10 (s, 1H), 2.66–2.30 (m, 1H), 2.19–2.06 (m, 1H), 1.84 (d, J = 13.5 Hz, 1H), 1.77–1.51 (m, 2H), 1.47–1.30 (m, 1H). 13C NMR (101 MHz, CDCl3) δ: 214.7, 148.3, 147.4, 127.8, 126.5, 123.3, 73.9, 57.1, 42.6, 30.7, 27.5, 24.6. HPLC analysis: Daicel Chiralpak AD-H, hexane/i-PrOH 90:10, flow rate 1.0 mL/min.
3.3.2. 2-(Hydroxy(3-nitrophenyl)methyl)cyclohexanone (6b)
Following the general procedure for the aldol reaction, 30 mg of 3-nitrobenzaldehyde (1.19 mmol) and 0.10 mL of cyclohexanone (0.90 mmol) were placed in a reaction vessel. To this mixture, 7.8 mg of the catalyst (0.01 mmol) dissolved in 0.3 mL of DMSO was added. The reaction afforded 7.4 mg of a white solid (15% yield). 1H NMR (400 MHz, CDCl3) δ 8.22 (s, 1H), 8.16 (dd, J = 8.2, 1.2 Hz, 1H), 7.68 (d, J = 7.7 Hz, 1H), 7.53 (t, J = 7.9 Hz, 1H), 4.90 (dd, J = 8.5, 2.2 Hz, 1H), 4.15 (d, J = 2.8 Hz, 1H), 2.69–2.58 (m, 1H), 2.55–2.46 (m, 1H), 2.43–2.33 (m, 1H), 2.12 (ddd, J = 12.6, 5.8, 2.9 Hz, 1H), 1.84 (d, J = 13.5 Hz, 1H). 13C NMR (101 MHz, CDCl3) δ 214.7, 148.1, 143.2, 133.1, 129.2, 122.7, 121.9, 73.9, 57.0, 42.5, 30.6, 27.5, 24.5. HPLC (Daicel Chiralpak AD-H, hexane/i-PrOH 95:5, flow rate 0.8 mL/min).
3.3.3. 2-(Hydroxy(2-nitrophenyl)methyl)cyclohexanone (6c)
Following the general procedure for the aldol reaction, 2-nitrobenzaldehyde (30 mg, 1.19 mmol) and cyclohexanone (0.10 mL, 0.90 mmol) were placed in 0.3 mL of DMSO. The catalyst (7.8 mg, 0.01 mmol) was then added, and the reaction mixture was stirred under the established conditions. The product was obtained as a white solid (6 mg, 12% yield). 1H NMR (400 MHz, CDCl3) δ: 7.85 (d, J = 8.2 Hz, 1H), 7.77 (d, J = 7.6 Hz, 1H), 7.64 (t, J = 7.6 Hz, 1H), 7.44 (dd, J = 11.2, 4.3 Hz, 1H), 5.45 (d, J = 7.1 Hz, 1H), 2.76 (dt, J = 12.7, 6.5 Hz, 1H), 2.45 (d, J = 13.6 Hz, 1H), 2.34 (td, J = 13.3, 6.2 Hz, 1H), 2.10 (ddd, J = 9.0, 5.7, 2.8 Hz, 1H), 1.85 (dd, J = 13.1, 2.5 Hz, 1H), 1.79–1.54 (m, 4H). 13C NMR (101 MHz, CD3OD) δ: 214.8, 148.6, 136.5, 133.0, 128.9, 128.3, 124.0, 69.7, 57.2, 42.7, 31.0, 27.7, 24.9. HPLC analysis: Daicel Chiralpak AD-H, hexane/i-PrOH 90:10, flow rate 0.8 mL/min.
3.4. General Procedure for the Heck Coupling Reaction
In a round-bottom flaesk equipped with magnetic stirring, 1.0 equiv of the corresponding aryl halide, 5.0 equiv of 2-cyclohexen-1-one, and 10 mol% of the catalyst were dissolved in a 1:1 mixture of H2O/DMF. To this solution, DIEA (3.0 equiv) was added. The reaction mixture was subjected to microwave irradiation in a CEM reactor at 100 W for 60 min. After completion, the product was extracted with EtOAc (3 × 20 mL). The combined organic layers were dried over anhydrous Na2SO4 and concentrated under reduced pressure using a rotary evaporator. The crude products were purified by column chromatography using hexane/EtOAc (80:20) as the eluent.
3.4.1. 3-(4-Hydroxyphenyl)cyclohex-2-en-1-one (7a)
Following the general procedure for the arylation of 2-cyclohexen-1-one, 4-iodophenol (40 mg, 1.8 mmol), 2-cyclohexen-1-one (0.90 mL, 9.1 mmol), and DIEA (0.06 mL, 5.44 mmol) were dissolved in a mixture of H2O/DMF (3 mL). The catalyst (2 mg, 0.5 mmol) was then added, and the reaction mixture was stirred under the established conditions. The crude product was purified to afford a white solid (0.022 g, 65% yield), mp 150–152 °C.
1H NMR (400 MHz, CDCl3) δ: 7.47 (d, J = 8.7 Hz, 2H, H-Ar), 7.06 (s, 1H, OH), 6.91 (d, J = 8.7 Hz, 1H, H-Ar), 6.42 (s, 1H, CH), 2.76 (t, J = 5.6 Hz, 2H, CH2), 2.55–2.43 (m, 2H, CH2), 2.19–2.05 (m, 1H, CH2). 13C NMR (100 MHz, CDCl3) δ: 201.0, 160.5, 158.3, 130.2, 127.9, 123.0, 115.8, 37.0, 27.8, 22.6. MS (EI) m/z: 188 (M+, 94%), 160 (100), 132 (65), 131 (60).
3.4.2. 3-(4-Methoxyphenyl)cyclohex-2-en-1-one (7b)
Following the general procedure for the arylation of 2-cyclohexen-1-one, iodoanisole (40 mg, 1.70 mmol), 2-cyclohexen-1-one (0.90 mL, 9.1 mmol), and DIEA (0.06 mL, 3.40 mmol) were dissolved in a mixture of H2O/DMF (3 mL). The catalyst (2 mg, 0.5 mmol) was then added, and the reaction mixture was stirred under the established conditions. The crude product was purified to afford a white solid (0.021 g, 61% y ield), mp 73–75 °C.
1H NMR (400 MHz, CDCl3) δ: 7.47 (d, J = 8.9 Hz, 2H, H-Ar), 6.93 (d, J = 9.7 Hz, 2H, H-Ar), 6.40 (t, J = 1.2 Hz, 1H, CH), 3.85 (s, 3H, CH3), 2.75 (td, J = 6.3, 1.2 Hz, 2H, CH2), 2.47 (td, J = 6.3, 1.2 Hz, 2H, CH2), 2.14 (m, 2H, CH2). 13C NMR (100 MHz, CDCl3) δ: 199.8, 161.1, 159.0, 130.7, 127.5, 123.6, 114.0, 55.3, 37.1, 27.7, 22.7. MS (EI) m/z: 202 (M+, 96%), 174 (100), 146 (70), 131 (47), 131 (59).