1. Introduction
Hydrogels are polymer materials with a three-dimensional network structure containing water, similar to soft tissues [
1].
Biopolymer hydrogels are derived from plants and animals, and are versatile materials typically assembled from entangled or crosslinked proteins and/or polysaccharides [
2]. These hydrogels are a promising class of materials as they can respond to external cues such as temperature, pH, and light [
3]. In addition, they have excellent functional properties, an amphiphilic nature, and are biocompatible and biodegradable, as well as being easily renewable for other uses and showing lower toxicity in comparison with synthetic polymers [
4].
Protein and polysaccharide hydrogels are generally induced via physical (heating, cooling, shear forces) and chemical (pH modulation, salt addition) induction techniques, or a mixed technique, to achieve the desired properties [
5]. Currently, diverse synthesis strategies enable the preparation of functional hydrogels with a range of applications [
1]. Hydrogels have numerous applications in medicine, agriculture, cosmetics, and the food industry. They are also used in personal care products, filters for water purification, and separation membranes [
6].
Hydrogels can be formulated by using a wide range of polymers, including those of food origin. The benefits of hydrogels made from food-grade biopolymers include their safety, low cost, and commercial availability. One possible way of creating food-based hydrogels is through the use of caseins, alone or in combination with other food-grade polymers [
7].
Four types of caseins are mainly present in bovine milk: αS1, αS2, β, and κ. These proteins adapt their structure to changes in environmental conditions and assemble to form colloidal aggregates, called casein micelles [
8].
Tetracaine hydrochloride, TH, is an amphiphilic compound that also possesses colloidal properties and is one of the most-used local anesthetic drugs. Since tetracaine is a poorly water-soluble compound, it is usually formulated as tetracaine hydrochloride [
9]. Considering the pharmacological properties of tetracaine, which can reduce pain caused by skin irritations from minor burns, we selected this compound for the development of a hydrogel for topical use.
In this study, different casein hydrogel formulations were prepared by changing the concentrations of the reagents. The hydrogels were characterized at the macroscopic level and their stability at different temperatures was evaluated.
2. Materials and Methods
2.1. Materials
Commercial micellar casein (CA) from bovine milk, glycerol (C3H8O3) (Gly), potassium alginate [(C12H14CaO12)n] (PAlg), and tetracaine hydrochloride (TH) were obtained commercially. A buffer of pH 10.6 [anhydrous potassium carbonate, K2CO3] (AnP) was prepared using deionized water.
2.2. Preparation of a Casein Hydrogel
The preparation of hydrogels was based on the previous literature [
10]. A casein solution (10 wt.%) with tetracaine was prepared by dissolving the protein in a tetracaine solution in potassium carbonate buffer (pH 10.6) at 60 °C under magnetic stirring. Then, glycerol and potassium alginate were added at different concentrations (
Table 1). The pH of the mixture was adjusted to 7 with acetic acid. After mixing the mixture for 30 min and cooling at 4 °C for 1 h, the hydrogels were obtained.
2.3. Caracterization of Hydrogels
The general appearance of the hydrogels was evaluated by analyzing the physical aspect, apparent density, pH, moisture content, swelling, and stability, considering the bibliography. The composition of tetracaine in the hydrogels was obtained via analysis with UV-Vis spectroscopy.
3. Results
To obtain hydrogels of adequate consistency, different formulations were tested, changing the concentration of TH (0.07 to 1.87%), CA (7.1 to 9.5%), AnP (83.8 to 88.56%), Gly (3.69 to 7.12%), and PAlg (0.19 to 0.40%).
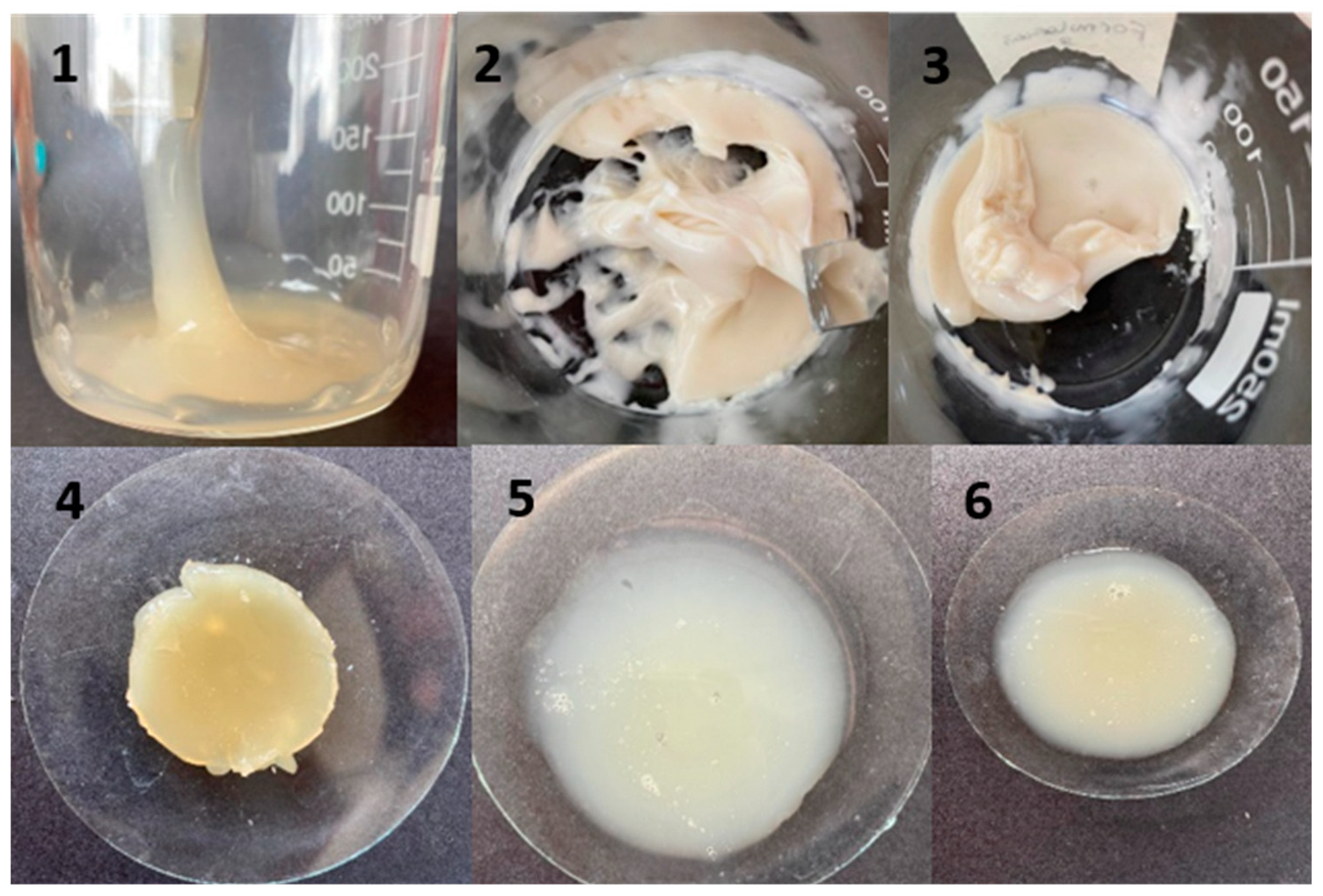
Figure 1 shows the obtained tetracaine hydrogels. The consistency of these formulations was evaluated at room temperature (20 °C) and at low temperature (4 °C). All formulations were initially viscous and firm; however, after 24 h, only formulations 1–4 maintained these properties. Considering that these were the best formulations, we continued with the characterization of these hydrogels.
Table 2 shows the results of the quality parameters of the casein hydrogels. Considering formulation 1 as the control, the other formulations did not show significant changes in their properties following the addition of tetracaine. In all cases, the incorporated tetracaine content was in line with the theoretical amount.
The swelling ratio of all hydrogels varied from 4.2 to 5.0 after 24 h, indicating that they are suitable for topical treatment. This result is consistent with that obtained by [
11].
In addition, release assays were performed in a phosphate buffer pH = 7.5, in which it was possible to evaluate that tetracaine is completely released from the hydrogels in less than one hour of testing.
4. Conclusions
In this work, casein, potassium alginate, glycerol, and tetracaine hydrochloride were used to formulate hydrogels. In these formulations, the apparent density was approximately 0.5, the pH was approximately 7, the moisture content was approximately 90%, and the swelling was approximately 4.5%. The optimal formulation was composed of tetracaine hydrochloride at 1%, casein at 2%, glycerol at 50%, and sodium alginate at 4%; therefore, it can be concluded that this combination provides the desired properties for the hydrogel. These results suggest that tetracaine hydrogel shows potential for use as an anesthetic in medicine.
Author Contributions
Methodology, Y.S.A., M.C.A. and L.G.G.; writing preparation, V.A.G.; writing—review C.A.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by UNIVERSIDAD TECNOLÓGICA NACIONAL, grant number PAPPSF0008588.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wang, X.; Zhang, H.J.; Yang, Y.; Chen, Y.; Zhu, X.; You, X. Biopolymer-based self-healing hydrogels: A short review. Giant 2023, 16, 100188. [Google Scholar] [CrossRef]
- Mahmood, A.; Patel, D.; Hickson, B.; DesRochers, J.; Hu, X. Recent Progress in Biopolymer-Based Hydrogel Materials for Biomedical Applications. Intern. J. Mol. Sci. 2022, 23, 1415. [Google Scholar] [CrossRef]
- Katyal, P.; Mahmoudinobar, F.; Montclare, J.K. Recent Trends in Peptide and Protein-based Hydrogels. Curr. Opin. Struct. Biol. 2020, 63, 97–105. [Google Scholar] [CrossRef]
- Abaee, A.; Mohammadian, M.; Jafari, S.M. Whey and soy protein-based hydrogels and nano-hydrogels as bioactive delivery systems. Trends Food Sci. Technol. 2017, 70, 69–81. [Google Scholar] [CrossRef]
- Hilal, A.; Florowska, A.; Florowski, T.; Wroniak, M. A Comparative Evaluation of the Structural and Biomechanical Properties of Food-Grade Biopolymers as Potential Hydrogel Building Blocks. Biomedicines 2022, 10, 2106. [Google Scholar] [CrossRef] [PubMed]
- Klein, M.; Poverenov, E. Natural biopolymer-based hydrogels for use in food and agriculture. J. Sci. Food Agric. 2020, 100, 2337–2347. [Google Scholar] [CrossRef]
- Nascimento Lima, L.G.; Casanova, F.; Silva Nogueira, N.F.; Novaes de Carvalho Teixeira, A.V.; Fernandes de Carvalho, A. Casein-based hydrogels: A mini-review. Food Chem. 2020, 314, 126063. [Google Scholar] [CrossRef]
- Corredig, M.; Nair, P.K.; Li, Y.; Eshpari, H.; Zhao, Z. Invited review: Understanding the behavior of caseins in milk concentrates. J. Dairy Sci. 2019, 102, 4772–4782. [Google Scholar] [CrossRef]
- Naved, A.; Rub, M.A.; Khan, A.; Alotaibi, M.M.; Asiri, A.M. Synergistic Interaction and Binding Efficiency of Tetracaine Hydrochloride (Anesthetic Drug) with Anionic Surfactants in the Presence of NaCl Solution Using Surface Tension and UV–Visible Spectroscopic Methods. Gels 2022, 8, 234. [Google Scholar] [CrossRef]
- Picchio, M.L.; Garro Linck, Y.; Monti, G.A.; Gugliotta, L.M.; Minari, R.J.; Alvarez Igarzabal, C.I. Casein films crosslinked by tannic acid for food packaging applications. Food Hydrocoll. 2018, 84, 424–434. [Google Scholar] [CrossRef]
- Hasan, M.M.; Uddin, M.F.; Zabin, N.; Shakil, M.S.; Alam, M.; Achal, F.J.; Begum, M.H.A.; Hossen, M.S.; Hasan, M.A.; Morshed, M.M. Fabrication and Characterization of Chitosan-Polyethylene Glycol (Ch-Peg) Based Hydrogels and Evaluation of Their Potency in Rat Skin Wound Model. Int. J. Biomater. 2021, 2021, 4877344. [Google Scholar] [CrossRef] [PubMed]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).