Synthesis of Peptide–Nonsteroidal Anti-Inflammatory Drug Hybrid Compounds and Their Selectivity in Inhibiting the Dual Cyclooxygenase-2 (COX)–5-Lipoxygenase (LOX) System: A Docking and Molecular Dynamics Study †
Abstract
1. Introduction
2. Results and Discussion
3. Experimental Section
3.1. General
3.2. Computational Details
3.3. General Procedure for Coupling
3.4. Spectral Data for Best Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dinarello, C.A. Proinflammatory cytokines. Chest 2000, 118, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Passos, F.R.S.; Heimfarth, L.; Monteiro, B.S.; Correa, C.B.; de Moura, T.R.; de Souza Araújo, A.A.; Martins-Filho, P.R.; Quintans-Júnior, L.J.; Quintans, J.D.S.S. Oxidative stress and inflammatory markers in patients with COVID-19: Potential role of RAGE, HMGB1, GFAP and COX-2 in disease severity. Int. Immunopharmacol. 2022, 104, 108502–108509. [Google Scholar] [CrossRef] [PubMed]
- Ayola-Serrano, N.C.; Roy, N.; Fathah, Z.; Anwar, M.M.; Singh, B.; Ammar, N.; Sah, R.; Elba, A.; Utt, R.S.; Pecho-Silva, S.; et al. The role of 5-lipoxygenase in the pathophysiology of COVID-19 and its therapeutic implications. Inflamm. Res. 2021, 70, 877–889. [Google Scholar] [CrossRef]
- Hoxha, M. A systematic review on the role of eicosanoid pathways in rheumatoid arthritis. Adv. Med. Sci. 2018, 63, 22–29. [Google Scholar] [CrossRef]
- Almutairi, K.; Nossent, J.; Preen, D.; Keen, H.; Inderjeeth, C. The global prevalence of rheumatoid arthritis: A meta-analysis based on a systematic review. Rheumatol. Int. 2021, 41, 863–877. [Google Scholar] [CrossRef]
- Rudrapal, M.; Eltayeb, W.A.; Rakshit, G.; El-Arabey, A.A.; Khan, J.; Aldosari, S.M.; Alshehri, B.; Abdalla, M. Dual synergistic inhibition of COX and LOX by potential chemicals from Indian daily spices investigated through detailed computational studies. Sci. Rep. 2023, 13, 8656. [Google Scholar] [CrossRef]
- Che, X.H.; Chen, C.L.; Ye, X.L.; Weng, G.B.; Guo, X.Z.; Yu, W.Y.; Tao, X.; Chen, Y.C.; Chen, X. Dual inhibition of COX-2/5-LOX blocks colon cancer proliferation, migration and invasion in vitro. Oncol. Rep. 2016, 35, 1680–1688. [Google Scholar] [CrossRef]
- Rajakrishnan, V.; Manoj, V.R.; Rao, G.S. Computer-aided, rational design of a potent and selective small peptide inhibitor of cyclooxygenase 2 (COX2). J. Biomol. Struct. Dyn. 2008, 25, 535–542. [Google Scholar] [CrossRef]
- Hong, Y.I.N.; PAN, X.C.; WANG, S.K.; YANG, L.G.; SUN, G.J. Protective effect of wheat peptides against small intestinal damage induced by non-steroidal anti-inflammatory drugs in rats. J. Integr. Agric. 2014, 13, 2019–2027. [Google Scholar]
- Mariniello, D.F.; Allocca, V.; D’Agnano, V.; Villaro, R.; Lanata, L.; Bagnasco, M.; Bianco, A.; Perrotta, F. Strategies tackling viral replication and inflammatory pathways as early pharmacological treatment for SARS-CoV-2 infection: Any potential role for ketoprofen lysine salt? Molecules 2022, 27, 8919. [Google Scholar] [CrossRef]
- Jorge-Aarón, R.M.; Rosa-Ester, M.P. N-acetylcysteine as a potential treatment for COVID-19. Future Microbiol. 2020, 15, 959–962. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, R.R.; Padhy, B.M.; Das, S.; Meher, B.R. Therapeutic potential of N-acetyl cysteine (NAC) in preventing cytokine storm in COVID-19: Review of current evidence. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 2802–2807. [Google Scholar] [PubMed]
- Tiwari, A.D.; Panda, S.S.; Girgis, A.S.; Sahu, S.; George, R.F.; Srour, A.M.; La Starza, B.; Asiri, A.M.; Hall, C.D.; Katritzky, A.R. Microwave assisted synthesis and QSAR study of novel NSAID acetaminophen conjugates with amino acid linkers. Org. Biomol. Chem. 2014, 12, 7238–7249. [Google Scholar] [CrossRef] [PubMed]
- Moreira, R.; Jervis, P.J.; Carvalho, A.; Ferreira, P.M.; Martins, J.A.; Valentão, P.; Andrade, P.B.; Pereira, D.M. Biological evaluation of naproxen–dehydrodipeptide conjugates with self-hydrogelation capacity as dual LOX/COX inhibitors. Pharmaceutics 2020, 12, 122–140. [Google Scholar] [CrossRef]
- Li, J.; Kuang, Y.; Gao, Y.; Du, X.; Shi, J.; Xu, B. D-amino acids boost the selectivity and confer supramolecular hydrogels of a nonsteroidal anti-inflammatory drug (NSAID). J. Am. Chem. Soc. 2013, 135, 542–545. [Google Scholar] [CrossRef]
- Li, J.; Kuang, Y.; Shi, J.; Gao, Y.; Zhou, J.; Xu, B. The conjugation of nonsteroidal anti-inflammatory drugs (NSAID) to small peptides for generating multifunctional supramolecular nanofibers/hydrogels. Beilstein J. Org. Chem. 2012, 9, 908–917. [Google Scholar] [CrossRef]
- Elhenawy, A.A.; Al-Harbi, L.M.; Moustafa, G.O.; El-Gazzar, M.A.; Abdel-Rahman, R.F.; Salim, A.E. Synthesis, comparative docking, and pharmacological activity of naproxen amino acid derivatives as possible anti-inflammatory and analgesic agents. Drug Des. Dev. Ther. 2019, 13, 1773–1790. [Google Scholar] [CrossRef]
- Jiménez-Cruz, J.C.; Guzmán-Mejía, R.; Juaristi, E.; Sánchez-Antonio, O.; García-Revilla, M.A.; González-Campos, J.B.; Aviña-Verduzco, J. Preparation of aromatic γ-hydroxyketones by means of Heck coupling of aryl halides and 2, 3-dihydrofuran, catalyzed by a palladium (ii) glycine complex under microwave irradiation. New J. Chem. 2020, 44, 13382–13392. [Google Scholar] [CrossRef]
- Chaudhary, N.; Aparoy, P. Application of per-residue energy decomposition to identify the set of amino acids critical for in silico prediction of COX-2 inhibitory activity. Heliyon 2020, 6, e04944. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Phillips, J.C.; Hardy, D.J.; Maia, J.D.; Stone, J.E.; Ribeiro, J.V.; Bernardi, R.C.; Buch, R.; Fiorin, G.; Hénin, J.; Jiang, W.; et al. Scalable molecular dynamics on CPU and GPU architectures with NAMD. J. Chem. Phys. 2020, 153, 044130. [Google Scholar] [CrossRef] [PubMed]
- Solís-Hernández, M.D.J.; Palomares-Báez, J.P.; Herrera-Bucio, R.; Chacón-García, L.; Navarro-Santos, P. Derivates of 1, 6-dihyadroazaazulenes as inhibitors of tyrosine kinases BCR-ABL1 wild type and mutant T315I: A molecular dynamics approach. J. Biomol. Struct. Dyn. 2023, 2023, 2279274. [Google Scholar] [CrossRef] [PubMed]
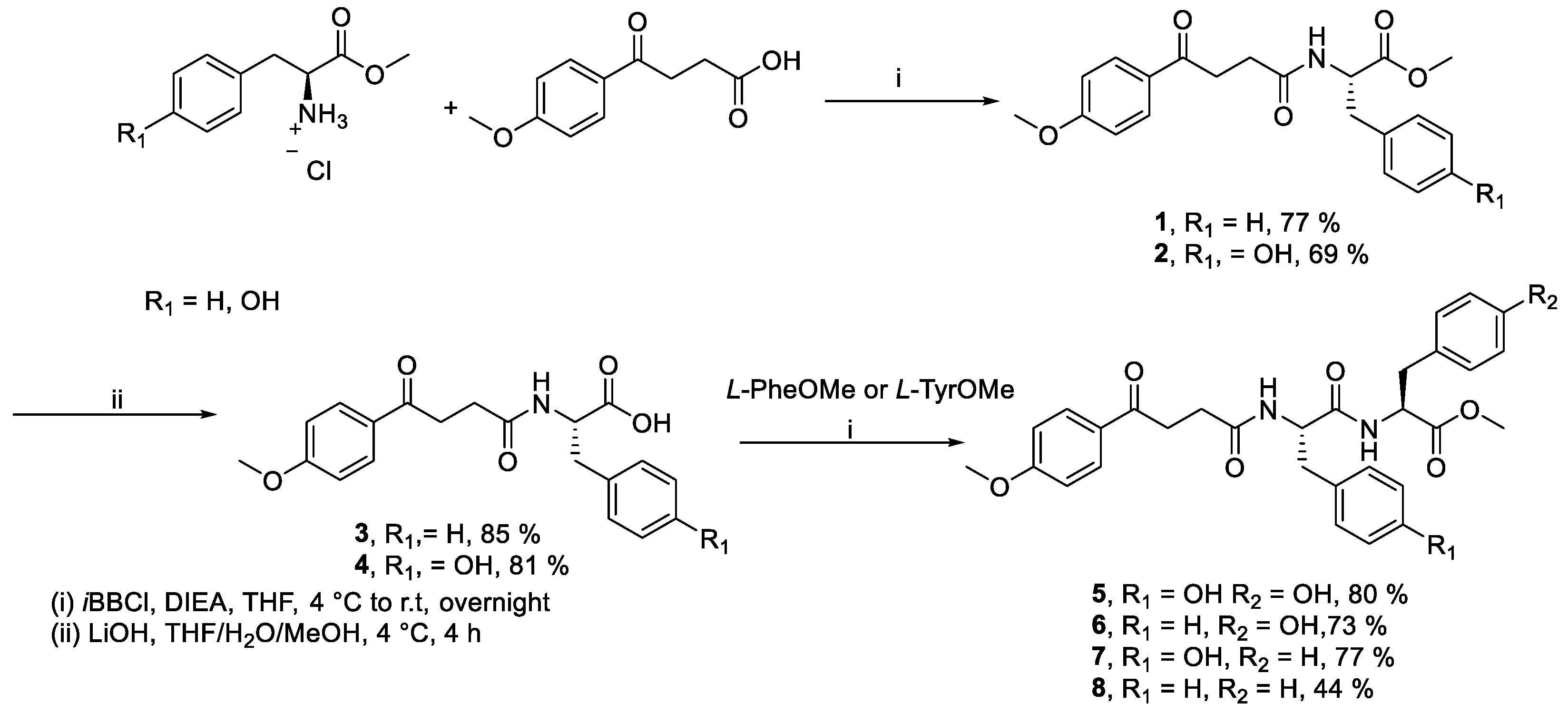
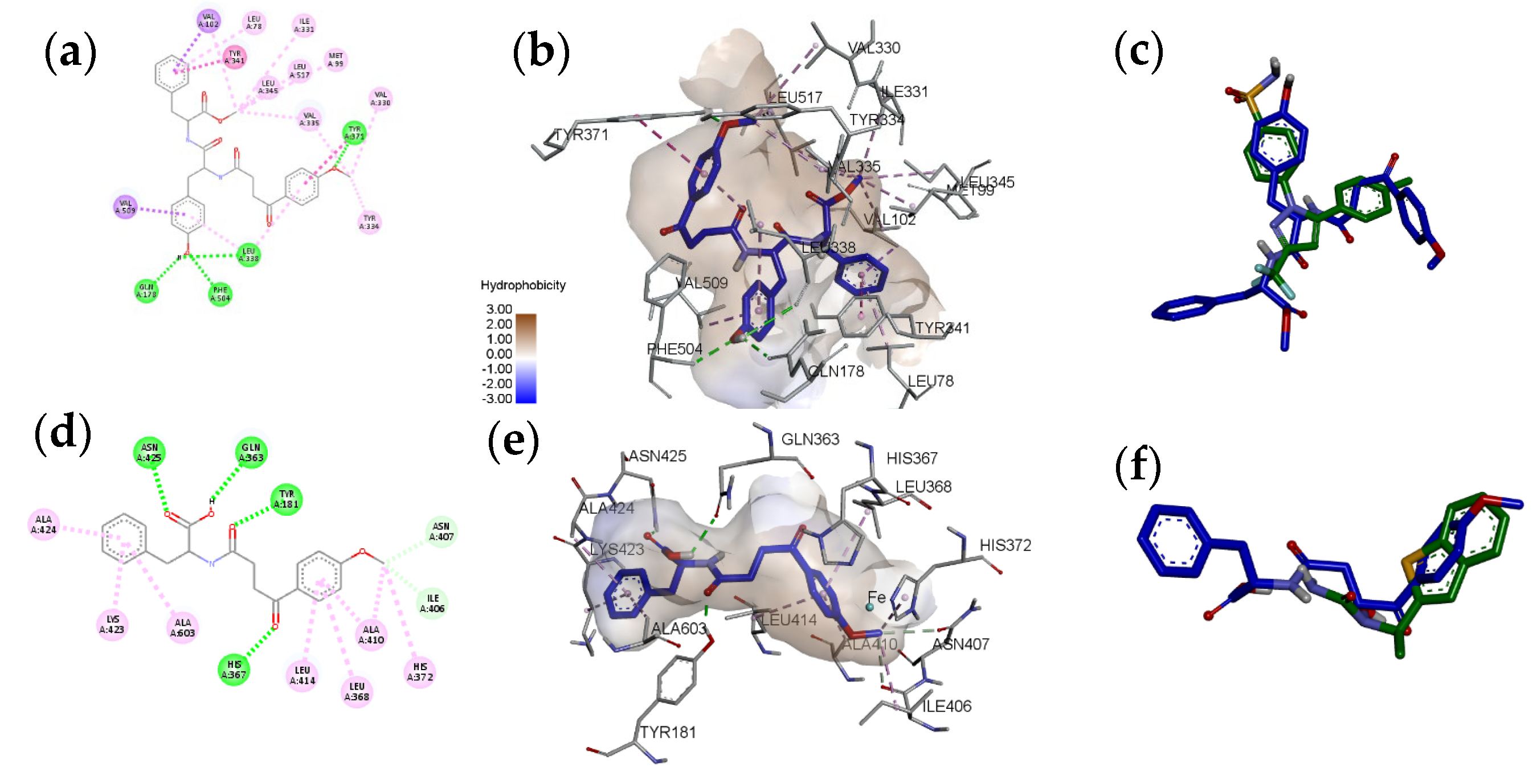
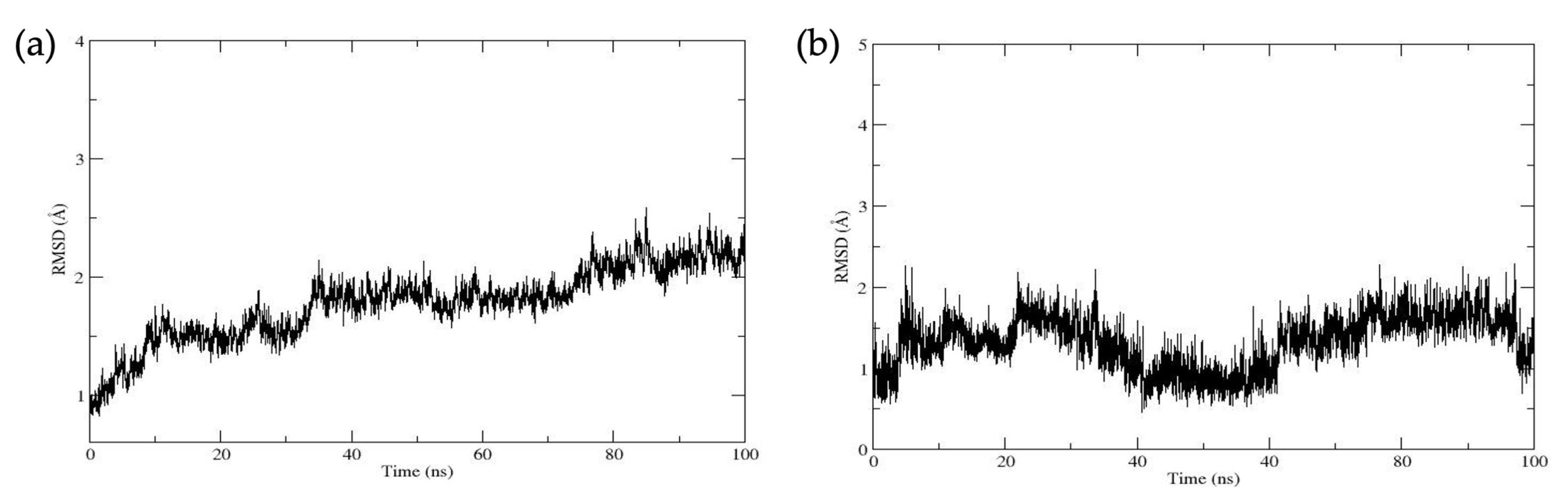
| Compound | ||||||||
|---|---|---|---|---|---|---|---|---|
| Entry | Protein | Reference | 3 | 4 | 5 | 6 | 7 | 8 |
| 1 | COX2 (kcal/mol) | −11.01 (celecoxib) | −10.38 | −9.49 | −10.09 | −11.10 | −11.01 | −10.57 |
| 2 | 5LOX (kcal/mol) | −7.83 (zileuton) | −9.06 | −9.05 | −6.89 | −9.2 | −7.3 | −8.90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiménez-Cruz, J.C.; Guzmán-Mejía, R.; Navarro-Santos, P.; García-Gutiérrez, H.A.; Ontiveros-Rodríguez, J.C.; Herrera-Bucio, R.; Aviña-Verduzco, J.A. Synthesis of Peptide–Nonsteroidal Anti-Inflammatory Drug Hybrid Compounds and Their Selectivity in Inhibiting the Dual Cyclooxygenase-2 (COX)–5-Lipoxygenase (LOX) System: A Docking and Molecular Dynamics Study. Chem. Proc. 2024, 16, 95. https://doi.org/10.3390/ecsoc-28-20256
Jiménez-Cruz JC, Guzmán-Mejía R, Navarro-Santos P, García-Gutiérrez HA, Ontiveros-Rodríguez JC, Herrera-Bucio R, Aviña-Verduzco JA. Synthesis of Peptide–Nonsteroidal Anti-Inflammatory Drug Hybrid Compounds and Their Selectivity in Inhibiting the Dual Cyclooxygenase-2 (COX)–5-Lipoxygenase (LOX) System: A Docking and Molecular Dynamics Study. Chemistry Proceedings. 2024; 16(1):95. https://doi.org/10.3390/ecsoc-28-20256
Chicago/Turabian StyleJiménez-Cruz, Juan C., Ramón Guzmán-Mejía, Pedro Navarro-Santos, Hugo A. García-Gutiérrez, Julio César Ontiveros-Rodríguez, Rafael Herrera-Bucio, and Judit A. Aviña-Verduzco. 2024. "Synthesis of Peptide–Nonsteroidal Anti-Inflammatory Drug Hybrid Compounds and Their Selectivity in Inhibiting the Dual Cyclooxygenase-2 (COX)–5-Lipoxygenase (LOX) System: A Docking and Molecular Dynamics Study" Chemistry Proceedings 16, no. 1: 95. https://doi.org/10.3390/ecsoc-28-20256
APA StyleJiménez-Cruz, J. C., Guzmán-Mejía, R., Navarro-Santos, P., García-Gutiérrez, H. A., Ontiveros-Rodríguez, J. C., Herrera-Bucio, R., & Aviña-Verduzco, J. A. (2024). Synthesis of Peptide–Nonsteroidal Anti-Inflammatory Drug Hybrid Compounds and Their Selectivity in Inhibiting the Dual Cyclooxygenase-2 (COX)–5-Lipoxygenase (LOX) System: A Docking and Molecular Dynamics Study. Chemistry Proceedings, 16(1), 95. https://doi.org/10.3390/ecsoc-28-20256






