Ultrasound-Assisted Extraction of Cannabidiol from Moroccan Cannabis sativa L. (Beldia) and Antioxidant Activity of Its Fractions †
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Extraction
2.2. Extraction Procedure
2.2.1. Petroleum Ether–Dichloromethane Extraction (PE-DI)
2.2.2. Methanol Extraction
2.2.3. Water Extraction
2.3. Determination of Extraction Yield
2.4. Determination of TPC and TFC and Antioxidant Assays
2.5. Column Chromatography
2.6. NMR and IR Analyses
3. Results
3.1. Extraction Yield
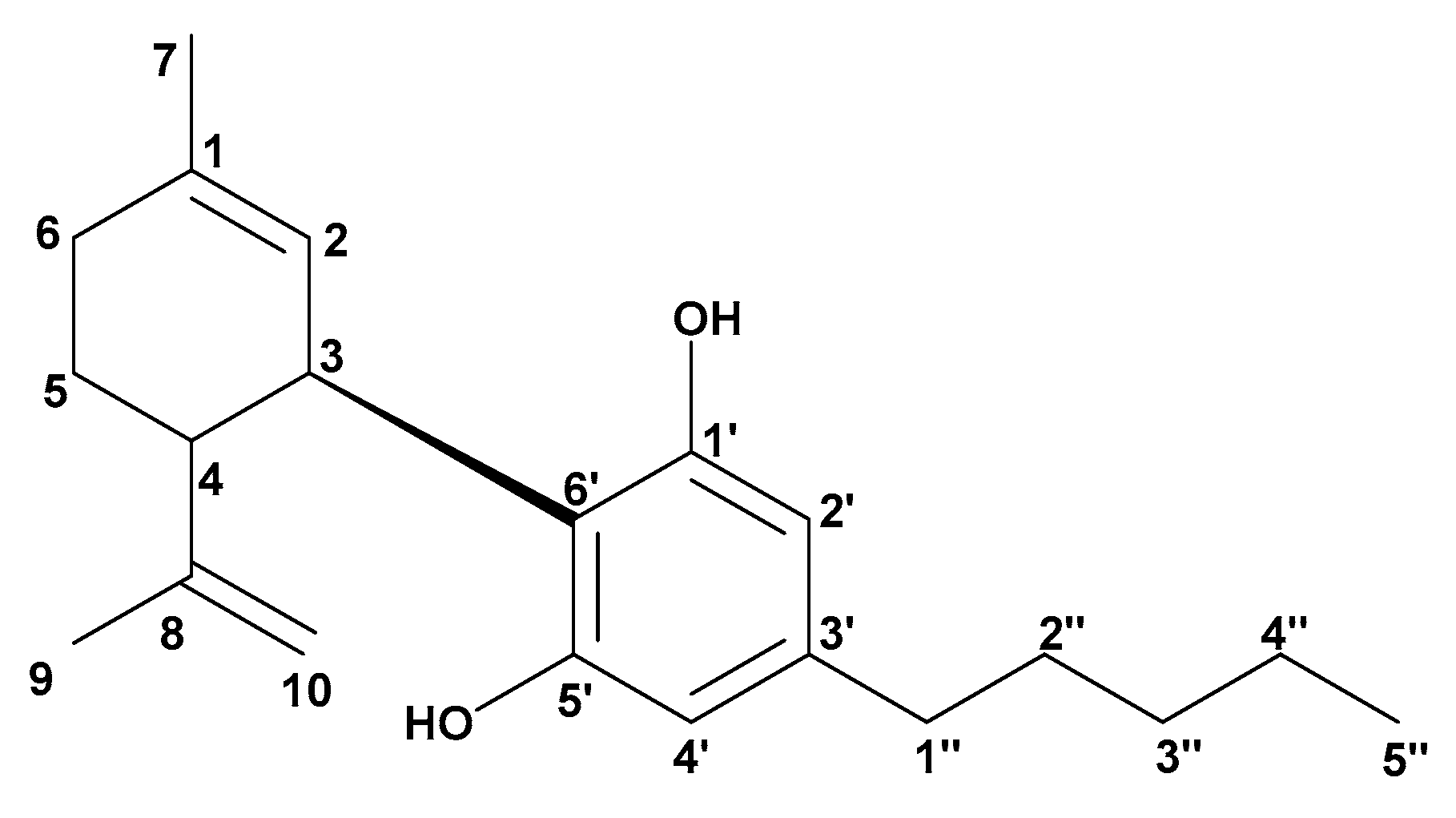
3.2. Separation and Purification of CBD Using Column Chromatography
3.3. Determination of TFC and TFC, DPPH Assay, and FRAP Assay
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sofowora, A.; Ogunbodede, E.; Onayade, A. The Role and Place of Medicinal Plants in the Strategies for Disease Prevention. Afr. J. Tradit. Complement. Altern. Med. 2013, 10, 210–229. [Google Scholar] [CrossRef]
- Pourseyed Lazarjani, M.; Torres, S.; Hooker, T.; Fowlie, C.; Young, O.; Seyfoddin, A. Methods for Quantification of Cannabinoids: A Narrative Review. J. Cannabis Res. 2020, 2, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Baswan, S.M.; Klosner, A.E.; Glynn, K.; Rajgopal, A.; Malik, K.; Yim, S.; Stern, N. Therapeutic Potential of Cannabidiol (CBD) for Skin Health and Disorders. Clin. Cosmet. Investig. Dermatol. 2020, 13, 927–942. [Google Scholar] [CrossRef] [PubMed]
- Legare, C.A.; Raup-Konsavage, W.M.; Vrana, K.E. Therapeutic Potential of Cannabis, Cannabidiol, and Cannabinoid-Based Pharmaceuticals. Pharmacology 2022, 107, 131–149. [Google Scholar] [CrossRef]
- Javadzadeh, Y.; Santos, A.; Aquilino, M.S.; Mylvaganam, S.; Urban, K.; Carlen, P.L. Cannabidiol Exerts Anticonvulsant Effects Alone and in Combination with Δ9-THC through the 5-HT1A Receptor in the Neocortex of Mice. Cells 2024, 13, 466. [Google Scholar] [CrossRef] [PubMed]
- Jones, N.A.; Hill, A.J.; Smith, I.; Bevan, S.A.; Williams, C.M.; Whalley, B.J.; Stephens, G.J. Cannabidiol Displays Antiepileptiform and Antiseizure Properties In Vitro and In Vivo. J. Pharmacol. Exp. Ther. 2010, 332, 569–577. [Google Scholar] [CrossRef]
- Kopustinskiene, D.M.; Masteikova, R.; Lazauskas, R.; Bernatoniene, J. Cannabis Sativa L. Bioactive Compounds and Their Protective Role in Oxidative Stress and Inflammation. Antioxidants 2022, 11, 660–667. [Google Scholar] [CrossRef]
- Lawag, I.L.; Nolden, E.S.; Schaper, A.A.M.; Lim, L.Y.; Locher, C. A Modified Folin-Ciocalteu Assay for the Determination of Total Phenolics Content in Honey. Appl. Sci. 2023, 13, 2135–2141. [Google Scholar] [CrossRef]
- Magalhaes, L.; Almeida, M.I.; Barreiros, L.; Reis, S.; Segundo, M. Automatic Aluminum Chloride Method for Routine Estimation of Total Flavonoids in Red Wines and Teas. Food Anal. Methods—Food. Anal. Meth. 2012, 5, 530–539. [Google Scholar] [CrossRef]
- Medini, F.; Fellah, H.; Ksouri, R.; Abdelly, C. Total Phenolic, Flavonoid and Tannin Contents and Antioxidant and Antimicrobial Activities of Organic Extracts of Shoots of the Plant Limonium delicatulum. J. Taibah Univ. Sci. 2014, 8, 216–224. [Google Scholar] [CrossRef]
- Govindappa, C.M.; Sadananda, T.S.; Chandrappa, C.P. Phytochemical Analysis of Loranthus Micranthus Extracts and Their in Vitro Antioxidant and Antibacterial Activities. J. Biol. Act. Prod. Nat. 2014, 4, 303–315. [Google Scholar] [CrossRef]
- Marzullo, P.; Foschi, F.; Coppini, D.A.; Fanchini, F.; Magnani, L.; Rusconi, S.; Luzzani, M.; Passarella, D. Cannabidiol as the Substrate in Acid-Catalyzed Intramolecular Cyclization. J. Nat. Prod. 2020, 83, 2894–2901. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Khan, M.; Al-hamoud, K.; Adil, S.F.; Shaik, M.R.; Alkhathlan, H.Z. Comprehensive Phytochemical Analysis of Various Solvent Extracts of Artemisia Judaica and Their Potential Anticancer and Antimicrobial Activities. Life 2022, 12, 1885–1892. [Google Scholar] [CrossRef]
- Onyebuchi, C.; Kavaz, D. Effect of Extraction Temperature and Solvent Type on the Bioactive Potential of Ocimum gratissimum L. Extracts. Sci. Rep. 2020, 10, 21760–21771. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.-W.; Lin, L.-G.; Ye, W.-C. Techniques for Extraction and Isolation of Natural Products: A Comprehensive Review. Chin. Med. 2018, 13, 20–29. [Google Scholar] [CrossRef]
- Izzo, L.; Castaldo, L.; Narváez, A.; Graziani, G.; Gaspari, A.; Rodríguez-Carrasco, Y.; Ritieni, A. Analysis of Phenolic Compounds in Commercial Cannabis Sativa L. Inflorescences Using UHPLC-Q-Orbitrap HRMS. Molecules 2020, 25, 631–639. [Google Scholar] [CrossRef] [PubMed]
- ElSohly, M.A.; Radwan, M.M.; Gul, W.; Chandra, S.; Galal, A. Phytochemistry of Cannabis Sativa L. In Phytocannabinoids: Unraveling the Complex Chemistry and Pharmacology of Cannabis sativa; Kinghorn, A.D., Falk, H., Gibbons, S., Kobayashi, J., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 1–36. ISBN 978-3-319-45541-9. [Google Scholar]
- Cásedas, G.; Moliner, C.; Maggi, F.; Mazzara, E.; López, V. Evaluation of Two Different Cannabis Sativa L. Extracts as Antioxidant and Neuroprotective Agents. Front. Pharmacol. 2022, 13, 1009868. [Google Scholar] [CrossRef] [PubMed]
- Barthlott, I.; Scharinger, A.; Golombek, P.; Kuballa, T.; Lachenmeier, D.W. A Quantitative 1H NMR Method for Screening Cannabinoids in CBD Oils. Toxics 2021, 9, 136–141. [Google Scholar] [CrossRef] [PubMed]
| Extract | TPC in mg EAG/g | TFC in mg QE/g | DDPH (IC50 in µg/mL) | FRAP (IC50 in µg/mL) |
|---|---|---|---|---|
| PE-DI fraction | 44.91 | 3.73 | 62.54 | 50.48 |
| Methanol fraction | 28.78 | 2.14 | 96.12 | 12.95 |
| Aqueous fraction | 5.91 | 0.17 | 252.72 | 204.97 |
| Ascorbic acid | - | - | 7.09 | 5.23 |
| BHT | - | - | 33.61 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serondo, H.U.; Bourgane, H.; El Kazzouli, S.; El Brahmi, N. Ultrasound-Assisted Extraction of Cannabidiol from Moroccan Cannabis sativa L. (Beldia) and Antioxidant Activity of Its Fractions. Chem. Proc. 2024, 16, 91. https://doi.org/10.3390/ecsoc-28-20253
Serondo HU, Bourgane H, El Kazzouli S, El Brahmi N. Ultrasound-Assisted Extraction of Cannabidiol from Moroccan Cannabis sativa L. (Beldia) and Antioxidant Activity of Its Fractions. Chemistry Proceedings. 2024; 16(1):91. https://doi.org/10.3390/ecsoc-28-20253
Chicago/Turabian StyleSerondo, Héritier Uwikunda, Hassana Bourgane, Saïd El Kazzouli, and Nabil El Brahmi. 2024. "Ultrasound-Assisted Extraction of Cannabidiol from Moroccan Cannabis sativa L. (Beldia) and Antioxidant Activity of Its Fractions" Chemistry Proceedings 16, no. 1: 91. https://doi.org/10.3390/ecsoc-28-20253
APA StyleSerondo, H. U., Bourgane, H., El Kazzouli, S., & El Brahmi, N. (2024). Ultrasound-Assisted Extraction of Cannabidiol from Moroccan Cannabis sativa L. (Beldia) and Antioxidant Activity of Its Fractions. Chemistry Proceedings, 16(1), 91. https://doi.org/10.3390/ecsoc-28-20253






