The Heterocyclization of 2-Imino-2H-chromeno-3-carbonitriles with Some N,N-Binucleophiles †
Abstract
1. Introduction
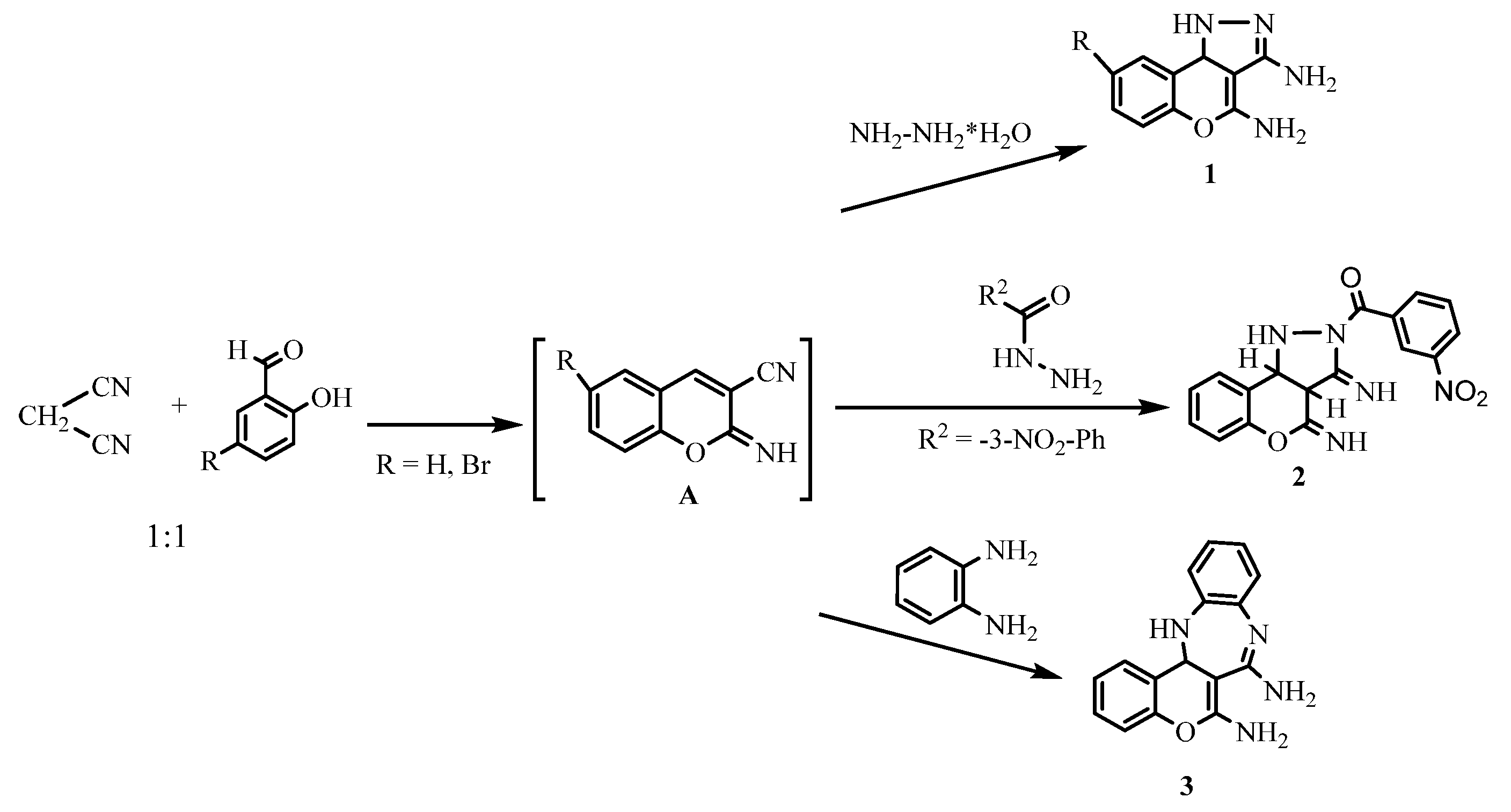
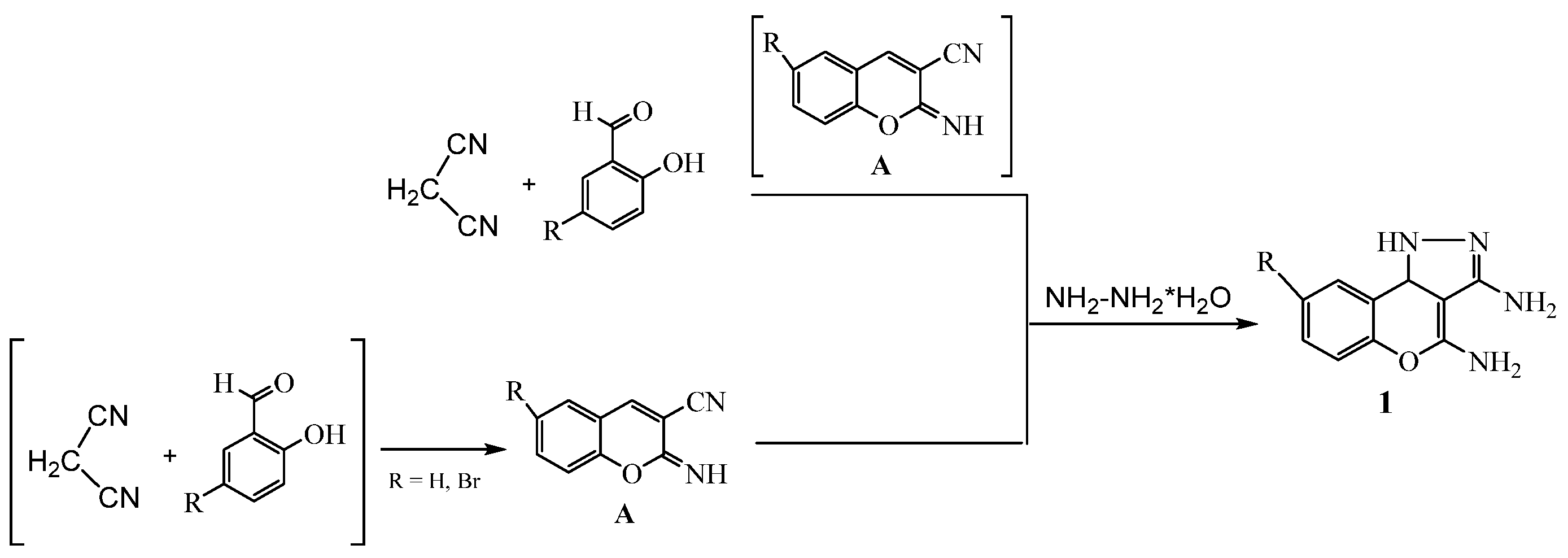
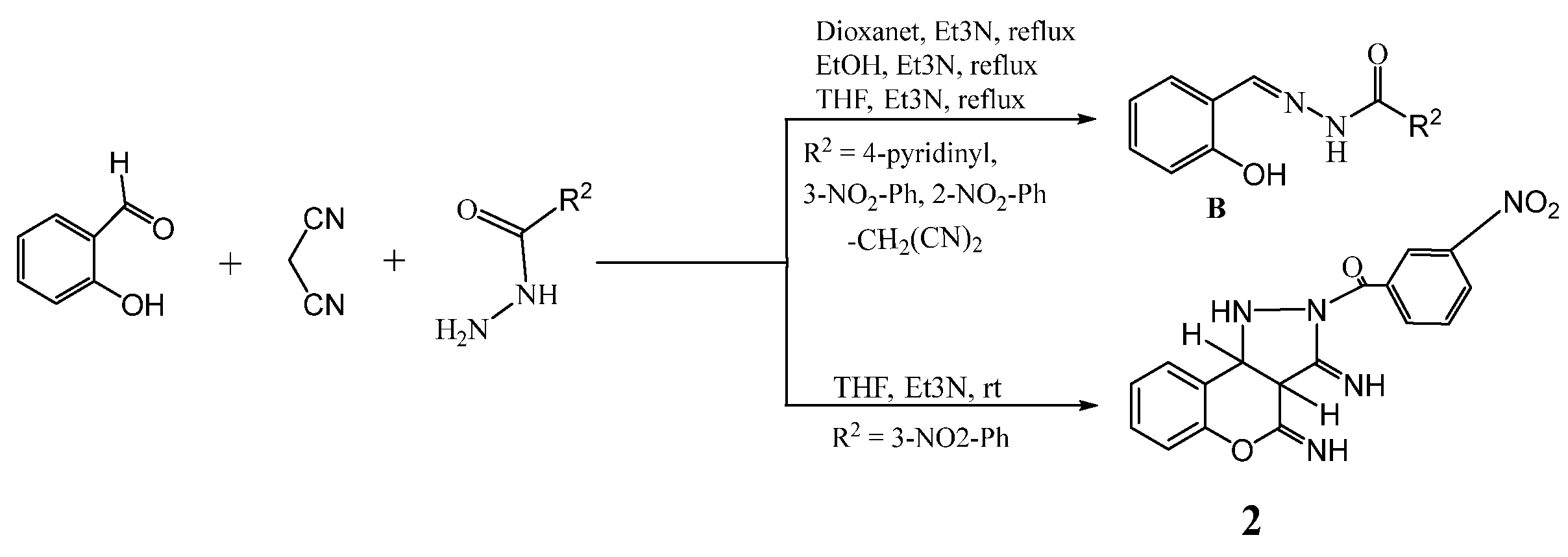
2. Results and Discussion
3. Experimental
3.1. General Information, Instrumentation, and Chemicals
3.2. Synthesis and Characterization of the Compounds
- 1,9b-dihydrochromeno[4,3-c]pyrazole-3,4-diamine 1a
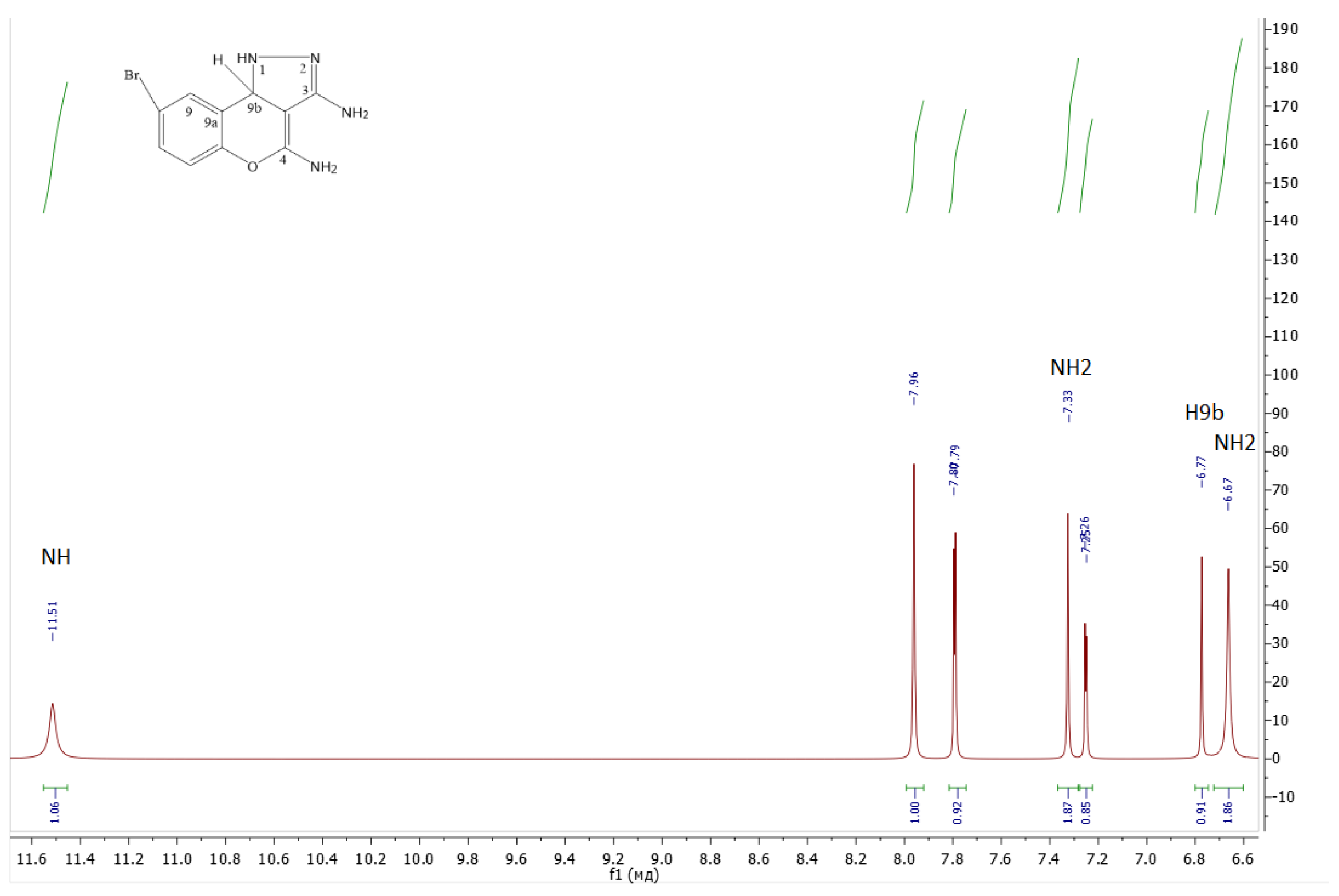
- 8-bromo-1,9b-dihydrochromeno[4,3-c]pyrazole-3,4-diamine 1b
- Yield: 69% (A), 75% (B).
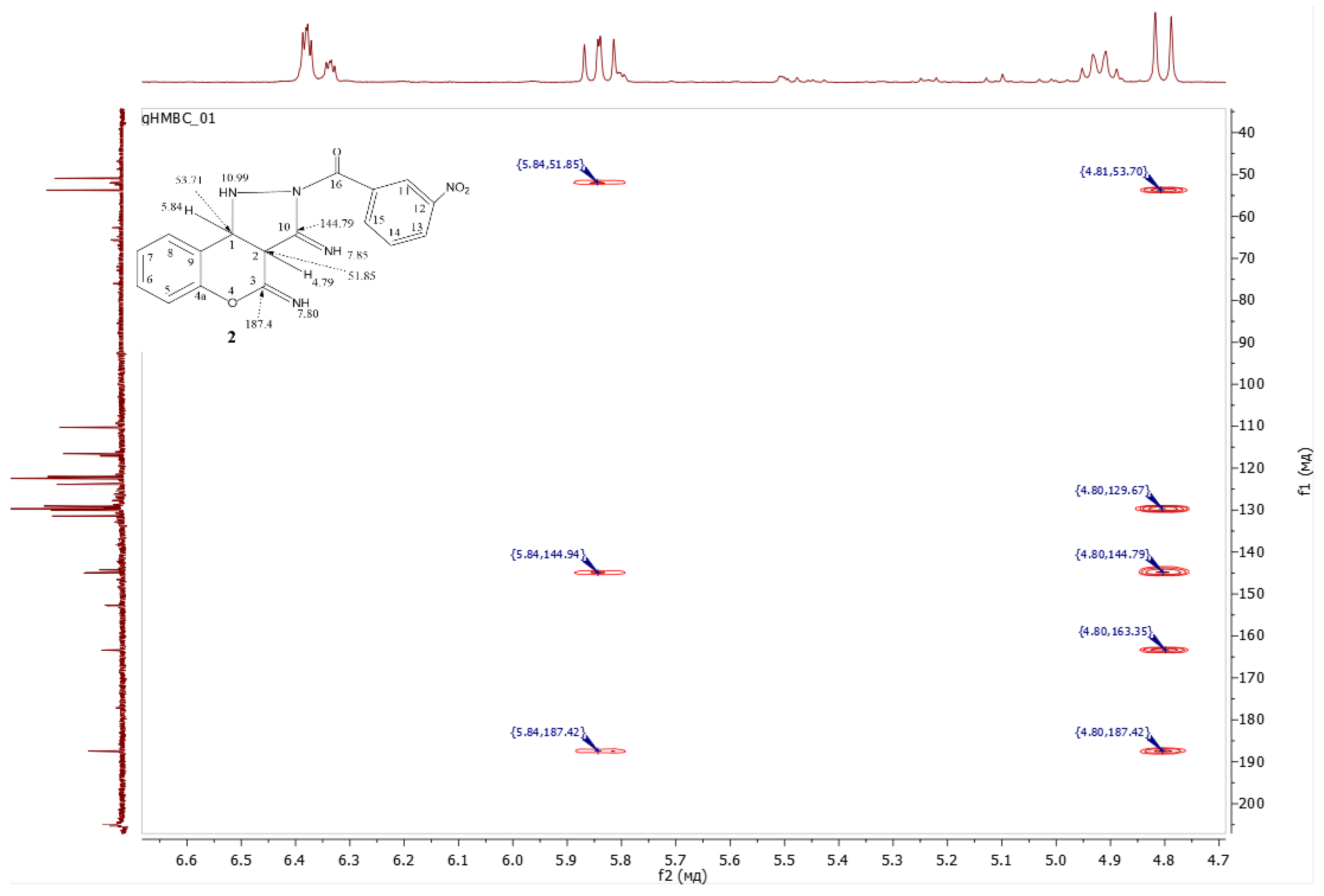
- (3,4-diimino-1,3a,4,9b-tetrahydrochromeno[4,3-c]pyrazol-2(3H)-yl)(3-nitrophenyl)methanone 2
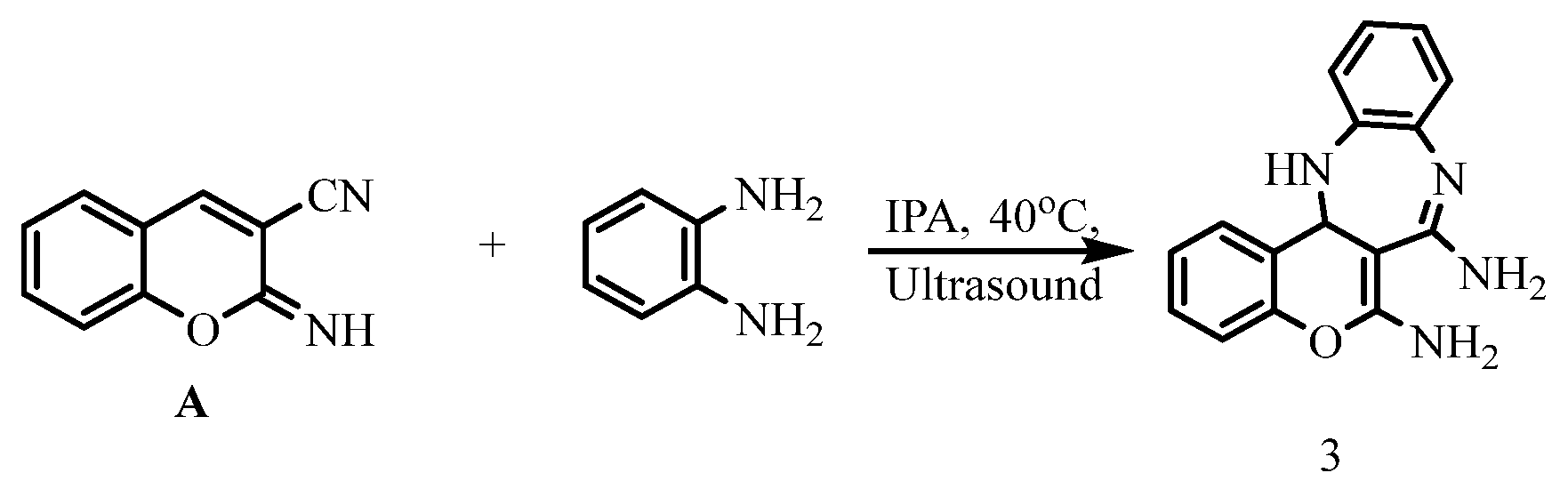
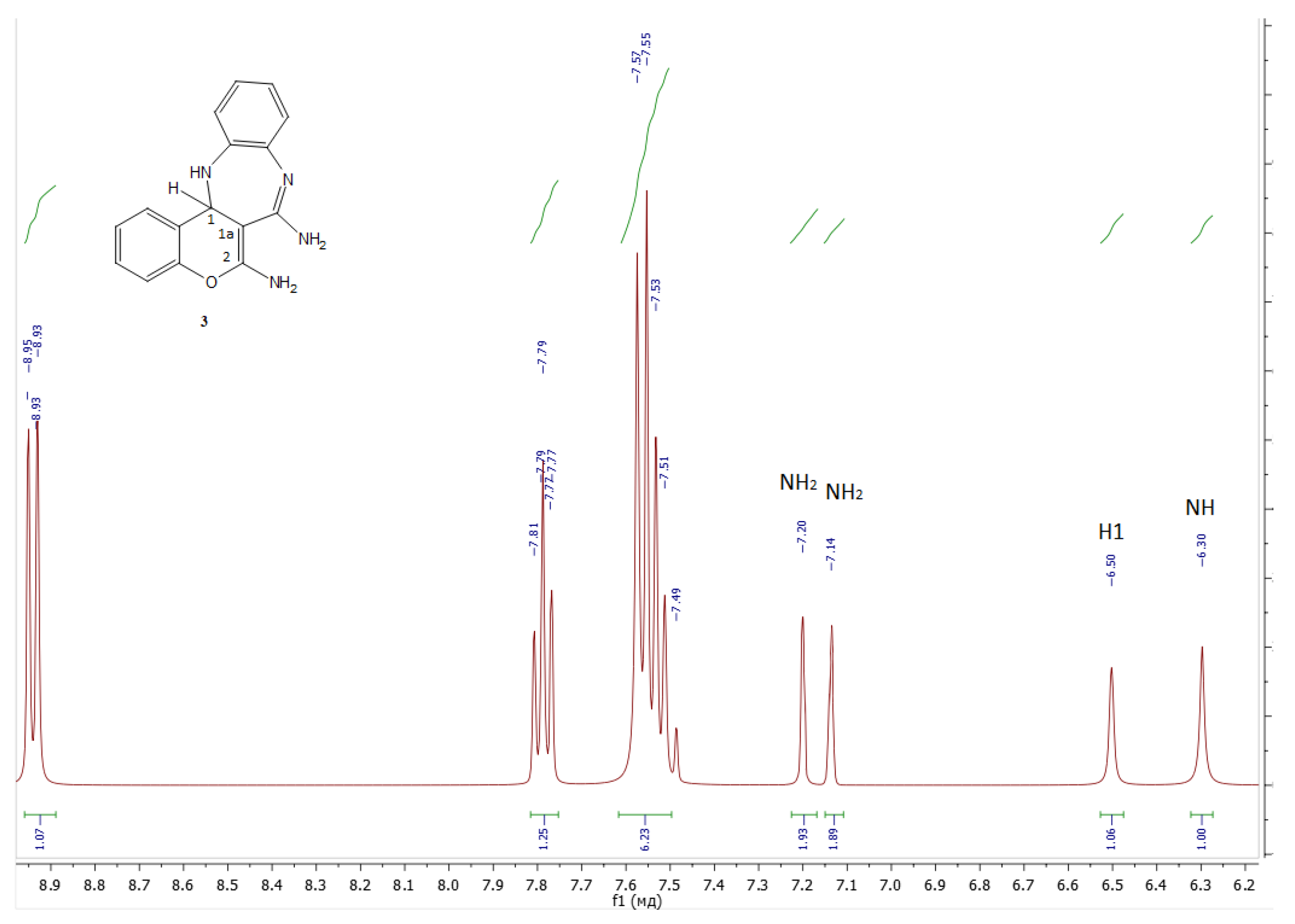
- 13,13a-dihydrobenzo[b]chromeno[4,3-e][1,4]diazepine-6,7-diamine 3
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yin, Y.; Hu, J.Q.; Wu, X.; Sha, S.; Wang, S.F.; Qiao, F.; Song, Z.C.; Zhu, H.L. Design, synthesis and biological evaluation of novel chromeno [4,3-c] pyrazol-4 (2H)-one derivates containing sulfonamido as potential PI3Kα inhibitors. Bioorg. Med. Chem. 2019, 27, 2261–2267. [Google Scholar] [CrossRef] [PubMed]
- Alshaye, N.A.; Ibrahim, M.A. Synthesis, characterization and biological evaluation of the novel chromenopyridothiazolopyrimidines and chromenopyridopyrimidothiazolo-pyrimidines. Synth. Commun. 2023, 53, 332–344. [Google Scholar] [CrossRef]
- Renuka, N.; Kumar, K.A. Synthesis and biological evaluation of fused pyrans bearing coumarin moiety as potent antimicrobial agents. Philipp. J. Sci. 2015, 144, 91–96. [Google Scholar]
- Hamdi, N.; Fischmeister, C.; Carmen Puerta, M.; Valerga, P. A rapid access to new coumarinyl chalcone and substituted chromeno [4, 3-c] pyrazol-4 (1 H)-ones and their antibacterial and DPPH radical scavenging activities. Med. Chem. Res. 2011, 20, 522–530. [Google Scholar] [CrossRef]
- Allehyani, E.S. Synthetic strategies, characterization and antimicrobial evaluation of novel angular heteroannulated furo [3,2-g] chromenes. Synth. Commun. 2023, 53, 1568–1578. [Google Scholar] [CrossRef]
- Fauziah, F.; Bakhtra, D.D.A. LC-HRMS Profile of Chemical Compounds in Penicillium citrinum XT6 Extract. IJPSM 2023, 8, 80–102. [Google Scholar] [CrossRef]
- Meshcheryakova, A.A.; Konstantinova, E.A.; Melkonyan, K.A.; Khrustaleva, A.A.; Sorokin, V.V. The Synthesis of Various 2-Imino-2H-chromene-3-carbonitrile Derivatives. Chem. Proc. 2023, 14, 42. [Google Scholar] [CrossRef]
- dos Santos, P.V.P.; Ribeiro, C.M.; Pavan, F.R.; Corbi, P.P.; Bergamini, F.R.; Carvalho, M.A.; D’Oliveria, K.A.; Cuin, A. Promising Ag (I) complexes with N-acylhydrazones from aromatic aldehydes and isoniazid against multidrug resistance in tuberculosis. J. Mol. Struct. 2021, 1234, 130193. [Google Scholar] [CrossRef]
- Elumalai, D.; Gnanasekaran, R.; Leelakrishnan, S.; Nachimuthu, G.; Kannan, T.; Paramasivam, T.P.; Jayabal, K. InCl3-Assisted Eco-Friendly Approach for N-Fused 1, 4-Dihydropyridine Scaffolds via Ring Opening Michael Addition of Cyclic Nitroketene and Iminocoumarin: Synthesis and DFT Studies. ChemistrySelect 2018, 3, 2070–2079. [Google Scholar] [CrossRef]
- Alizadeh, A.; Ghanbaripour, R.; Zhu, L.G. Recyclization of 2-Iminochromenes via Ketene Aminal Intermediates: One-Pot, Three-Component Reaction for Synthesis of 1, 4-Dihydropyridines-fused-1, 3-diazaheterocycles. Synth. Commun. 2013, 43, 2575–2582. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meshcheryakova, A.A.; Konstantinova, E.A.; Melkonyan, K.A.; Vidlatskaya, D.V.; Sorokin, V.V. The Heterocyclization of 2-Imino-2H-chromeno-3-carbonitriles with Some N,N-Binucleophiles. Chem. Proc. 2024, 16, 93. https://doi.org/10.3390/ecsoc-28-20246
Meshcheryakova AA, Konstantinova EA, Melkonyan KA, Vidlatskaya DV, Sorokin VV. The Heterocyclization of 2-Imino-2H-chromeno-3-carbonitriles with Some N,N-Binucleophiles. Chemistry Proceedings. 2024; 16(1):93. https://doi.org/10.3390/ecsoc-28-20246
Chicago/Turabian StyleMeshcheryakova, Anna A., Ekaterina A. Konstantinova, Karina A. Melkonyan, Daria V. Vidlatskaya, and Vitaliy V. Sorokin. 2024. "The Heterocyclization of 2-Imino-2H-chromeno-3-carbonitriles with Some N,N-Binucleophiles" Chemistry Proceedings 16, no. 1: 93. https://doi.org/10.3390/ecsoc-28-20246
APA StyleMeshcheryakova, A. A., Konstantinova, E. A., Melkonyan, K. A., Vidlatskaya, D. V., & Sorokin, V. V. (2024). The Heterocyclization of 2-Imino-2H-chromeno-3-carbonitriles with Some N,N-Binucleophiles. Chemistry Proceedings, 16(1), 93. https://doi.org/10.3390/ecsoc-28-20246





