Abstract
A particular interest in oncology has presented the migration of cancer cells, collective and individual, and the switch between these two types of motilities greatly complicates this disease and adds further complexity to therapeutic approaches. Intercellular connections play a prominent role in motility, especially E-cadherin and β-catenin markers. Unsaturated fatty acid 10HDA has not been investigated so far on the collective migration of colorectal carcinoma (CRC) cells and components of intercellular junctions. Our study highlights the prominent anti-migratory effects of 10H2DA on HCT-116 cells, obviously due to the significant increase in E-cadherin and β-catenin protein expression, which are crucial targets in cancer treatment, and therefore should be extensively analyzed. In conclusion, 10H2DA presents a valuable source of anticancer potential worthy of further investigation regarding the development of therapeutic strategies against CRC.
1. Introduction
Among cancers, colorectal cancer (CRC) represents one of the most significant health problems worldwide [1]. The main therapeutic approaches in cancer treatment, such as surgery, chemotherapy, and radiation, have not yielded satisfactory results so far due to the side effects they cause [2]. Therefore, medicine turns to their improvement by using natural products [3]. One of those is royal jelly (RJ) with prominent anticancer effects which are attributed to its active substance: 10-hydroxy-trans-2-decenoic (10H2DA).
Combating cancer is problematic because of the acquisition of migratory potential as a key moment for cancer cells, which enables their invasion and dissemination from primary sites to distant organs, and the establishment of lethal secondary foci. What is particularly interesting is the existence of two main types of migration of cancer cells: collective and individual. The collective type involves the movement of multiple cells as a single entity, while maintaining strong intercellular connections formed by cadherin–catenin complexes. Numerous studies have identified E-cadherin and β-catenin as good biomarkers for the prognosis of CRC and liver metastasis [4].
Considering that dietary habits are one of the factors for CRC formation and progression, and that RJ has been used as a dietary supplement for centuries, it is very important to analyze the effect of its bioactive molecule 10H2DA on CRC cell line HCT-116. This is of particular interest because no available research data revealed any 10H2DA activity regarding CRC and its anti-migratory potential on this type of cancer. All of the above led us to conduct such a study, and moreover, to investigate the exact molecular targets of 10H2DA; specifically, the effects of this component on the expression of E-cadherin and β-catenin in HCT-116 cells, which have not been reported so far.
2. Methods
Colorectal carcinoma cell line HCT-116, isolated from stage IV CRC, was purchased from the American Type Culture Collection (ATCC, Manassas, VI, USA). Cells were cultured according to standard culturing conditions, using Dulbecco’s Modified Eagle Medium (DMEM, Lonza, Switzerland) supplemented with 10% fetal bovine serum (FBS, Capricorn Scientific, Germany), and 100 U/mL penicillin/100 μg/mL streptomycin (Gibco, Invitrogen, Thermo Fisher Scientific, Waltham, MA, USA). Cells were maintained in a humidified atmosphere with 5% CO2 at 37 °C until 90% of confluence was reached.
10H2DA (purchased from TCI Chemicals, Tokyo, Japan) was diluted in DMEM and dimethyl sulfoxide (SERVA, Heidelberg, Germany) in order to obtain working concentrations of 10 and 100 µM. These two concentrations were applied for the two following assays as they already showed no cytotoxicity on HCT-116 cells [5].
The collective migratory capacity of these cells was assessed by using a wound-healing (Scratch) assay and detailed protocol was previously described in Jovanović et al. [5]. The migration of cells was monitored during 24 h of treatment using an inverted NICON Eclipse-Ti microscope (Nikon Instruments Inc., Melville, NY, USA). Micrographs were taken at 100× magnification and the motility rate was quantified by ImageJ software package (ver. 1.52a). The degree of cell migration is presented as relative wound space (in %) from two independent experiments performed in triplicate.
The protein expression of E-cadherin and β-catenin was determined by immunofluorescent method according to the procedure described previously [5]. Micrographs were obtained using Eclipse Ti (Nikon Instruments Inc.) inverted fluorescent microscope at 600× magnification and the resulting fluorescence intensity of the target protein in the cells was quantified by using the ImageJ software package [6]. The results are presented as relative fluorescence intensity per cell (in %) from two independent experiments performed in triplicate.
Data analyses were carried out in IBM SPSS Statistics, v. 17 (IBM Corp., Armonk, NY, USA) and the obtained results are expressed as the mean value ± standard error (S.E.) using Student’s t-test and one-way ANOVA for multiple comparisons to evaluate statistical significance.
3. Results
3.1. Antimigratory Effects
To explore the impact of 10H2DA on HCT-116 cells’ motility, the wound-healing method was applied. Our study highlights the prominent dose-dependent anti-migratory effects of 10H2DA 24 h after treatment (Figure 1). It is obvious that this treatment significantly slowed down the mobility of tested colorectal carcinoma cells when compared to the control (untreated) cells (Figure 1). Considering that this CRC cell line is already described as very aggressive, our findings point to the valuable anti-migratory potential of this unsaturated fatty acid.
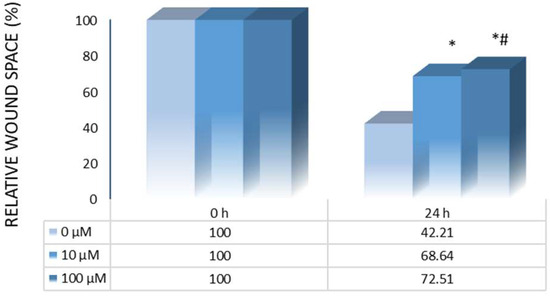
Figure 1.
Motility rate of control (untreated) and HCT-116 cells treated with 10H2DA after 24 h. * p < 0.05 is considered a statistically significant difference between treatment periods compared to 0 h, while # p < 0.05 is a statistically significant difference between treatment concentrations in a treated group for the same time period.
3.2. Protein Expression and Localization
To elucidate the mechanisms through which 10H2DA inhibits anti-migratory potential on the tested colorectal carcinoma cell line, an immunofluorescent staining was applied, and the two main components of intercellular junctions, E-cadherin and β-catenin, were labeled.
This study reveals an impact of 10H2DA treatment on investigated protein expression when the intensity of fluorescence was compared to the control values. Our results revealed the stimulatory effects of examined fatty acid on E-cadherin and β-catenin markers. However, only a higher 10H2DA concentration (100 µM) was able to significantly elevate the E-cadherin expression with its cellular localization in the cell cytoplasm and membrane area (Figure 2 and Figure 3a). Meanwhile, cytoplasmic β-catenin expression was heightened by both concentrations of this fatty acid (10 and 100 µM); however, its lower concentration was slightly more effective in this increase (Figure 2). Furthermore, the localization of cytoplasmic β-catenin in HCT-116 cells treated with 10H2DA was found predominantly concentrated at the cell membrane area (Figure 3b).
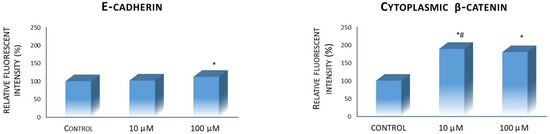
Figure 2.
E-cadherin and cytoplasmic β-catenin protein expression in control (untreated) and HCT-116 cells treated with 10H2DA 24 h after treatment. * p < 0.05 is considered a statistically significant difference between treatment periods compared to 0 h, while # p < 0.05 is a statistically significant difference between treatment concentrations in a treated group for the same time period.
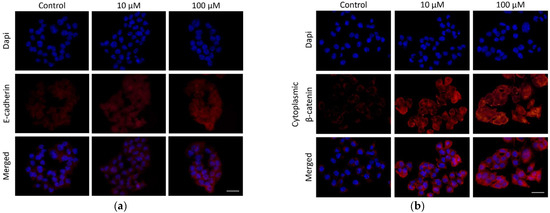
Figure 3.
Micrographs representing E-cadherin (a) and cytoplasmic β-catenin (b) protein expression and localization in control (untreated) and HCT-116 cells treated with 10H2DA, 24 h after treatment. Scale bar: 50 µm.
The increase in these two components of intercellular junction complexes and suppressed HCT-116 cells’ motility is obviously in tight association, implying their importance in CRC cell aggressiveness.
4. Discussion
Collective migration is one of two main types of cancer cell movement, presenting a particular problem in combating cancer. Intense communication between cells in this type of cancer behavior is performed via well-established intercellular junctions [7]. The majority of epithelial cancers migrate precisely in this collective way [8]. Transmembrane protein E-cadherin mediates these adhesive connections between epithelial cells [9], and its loss leads to weakened contact inhibition resulting in increased motility and invasiveness of colon cancer cells, as well as the advancement of cancer disease [10]. β-catenin mediates the binding of the cytoplasmic domain of E-cadherin to cytoskeletal actin filaments, thus affecting cellular movement [4]. During metastasis, these junctions are usually dysregulated in cancer cells, and β-catenin becomes localized in the nuclei of the cells, representing a key regulator of the Wnt signaling pathway, which, when activated, causes the proliferation and motility of cancer cells [11].
Nutrition is also observed as a responsible factor for the increased risk of colorectal carcinogenesis. Several studies demonstrated that RJ, which is vastly used as a dietary supplement, significantly inhibits the formation and growth of some types of tumors in vivo, suppresses angiogenesis, possesses estrogen and anti-migratory activity, and prevents the occurrence of metastases. These prominent anticancer effects are mainly attributed to unsaturated fatty acid 10H2DA, a specific and unique component of RJ, present only in this natural product, making this substance the marker of RJ’s quality [12].
Our study shows, for the first time, the inhibitory effects of 10H2DA on the collective migratory potential of colorectal carcinoma cell line HCT-116. This cell line is purposefully selected because it originates from an advanced stage of CRC and is observed as highly aggressive with very strong invasive and migratory characteristics [13]. That is precisely why the reported anti-migratory activity of 10H2DA in our study is very significant, designating this molecule as a powerful bioactive substance. It is obvious that its activity is based on increased E-cadherin and β-catenin protein levels, which most probably led to the reestablishment of loose intercellular junctions. Similar effects of this acid have been shown recently. Namely, the suppression of lung cancer cells’ migratory potential by 10H2DA was revealed to be due to the increase in E-cadherin and the suppression of N-cadherin, vimentin and SNAIL pro-migratory/invasive markers [12]. It is also known that other bee products, such as melittin, the main component of bee venom, can increase E-cadherin expression in pancreatic and liver cancer cells [14,15]. Also, 10H2DA showed estrogenic activity due to its strong affinity for binding to the estrogenic receptor β (ERβ), the predominant form of estrogen receptor in CRC [16,17]. Its activation leads to the regulation of many target genes, among which are E-cadherin and β-catenin [18]. Therefore, this could be the most probable mechanism through which this acid exerts its activity.
5. Conclusions
The present study results provide novel insights into 10H2DA for CRC treatment and shed light on potential strategies for modulating the expression patterns of intercellular junction complex markers. The results underscore the significant impact of E-cadherin and β-catenin on CRC motility and aggressiveness and 10H2DA emerges as a promising therapeutic candidate for inhibiting CRC migration by targeting these markers, thus offering potential avenues for modulating in CRC therapy.
Author Contributions
Conceptualization, D.Š. and M.M.J.; methodology, D.Š. and M.M.J.; software, D.N. and I.S.; validation, D.Š. and M.M.J.; formal analysis, D.A.; investigation, D.Š. and M.M.J.; resources, D.N. and I.S.; data curation, D.Š.; writing—original draft preparation, M.M.J.; writing—review and editing, D.Š.; visualization, M.M.J.; supervision, D.Š.; project administration, D.N. and I.S.; funding acquisition, D.Š., D.N. and I.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Science, Technological Development and Innovation of the Republic of Serbia, grant numbers 451-03-47/2023–01/200122, 175103 and 451-03-68/2023–14/200124.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hossain, M.S.; Karuniawati, H.; Jairoun, A.A.; Urbi, Z.; Ooi, J.; John, A.; Lim, Y.C.; Kibria, K.M.K.; Mohiuddin, A.K.M.; Ming, L.C.; et al. Colorectal cancer: A review of carcinogenesis, global epidemiology, current challenges, risk Factors, preventive and treatment strategies. Cancers 2022, 14, 1732. [Google Scholar] [CrossRef] [PubMed]
- Asma, S.T.; Acaroz, U.; Imre, K.; Morar, A.; Shah, S.R.A.; Hussain, S.Z.; Arslan-Acaroz, D.; Demirbas, H.; Hajrulai-Musliu, Z.; Istanbullugil, F.R.; et al. Natural products/bioactive compounds as a source of anticancer drugs. Cancers 2022, 14, 6203. [Google Scholar] [CrossRef] [PubMed]
- Miyata, Y.; Sakai, H. Anti-cancer and protective effects of royal jelly for therapy-induced toxicities in malignancies. Int. J. Mol. Sci. 2018, 19, 3270. [Google Scholar] [CrossRef] [PubMed]
- Christou, N.; Perraud, A.; Blondy, S.; Jauberteau, M.O.; Battu, S.; Mathonnet, M. The extracellular domain of E cadherin linked to invasiveness in colorectal cancer: A new resistance and relapses monitoring serum-bio marker? J. Cancer Res. Clin. Oncol. 2017, 143, 1177–1190. [Google Scholar] [CrossRef] [PubMed]
- Jovanović, M.M.; Šeklić, D.S.; Rakobradović, J.D.; Planojević, N.S.; Vuković, N.L.; Vukić, M.D.; Marković, S.D. Royal jelly and trans-10-hydroxy-2-decenoic acid inhibit migration and invasion of colorectal carcinoma cells. Food Technol. Biotechnol. 2022, 60, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Pijuan, J.; Barceló, C.; Moreno, D.F.; Maiques, O.; Sisó, P.; Marti, R.M.; Macià, A.; Panosa, A. In vitro cell migration, invasion, and adhesion assays: From cell imaging to data analysis front. Cell Dev. Biol. 2019, 7, 107. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.S.; Jiang, J.; Chen, B.J.; Wang, K.; Tang, Y.L.; Liang, X.H. Plasticity of cancer cell invasion: Patterns and mechanisms. Transl. Oncol. 2021, 14, 100899. [Google Scholar] [CrossRef] [PubMed]
- Moreno, M.R.; Stempor, P.A.; Bulgakova, N.A. Interactions and feedbacks in E-cadherin transcriptional regulation. Front. Cell Dev. Biol. 2021, 9, 701175. [Google Scholar]
- Mendonsa, A.M.; Na, T.Y.; Gumbiner, B.M. E-cadherin in contact inhibition and cancer. Oncogene 2018, 37, 4769–4780. [Google Scholar] [CrossRef] [PubMed]
- Vu, T.; Datta, P.K. Regulation of EMT in colorectal cancer: A culpritin metastasis. Cancers 2017, 9, 171. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.M.; Liu, S.B.; Luo, Y.H.; Xu, W.T.; Zhang, Y.; Zhang, T.; Xue, H.; Zuo, W.B.; Li, Y.N.; Lu, B.X.; et al. 10-HDA induces ROS-mediated apoptosis in A549 human lung cancer cells by regulating the MAPK, STAT3, NF-κB, and TGF-Β1 signaling pathways. BioMed Res. Int. 2020, 2020, 3042636. [Google Scholar] [CrossRef] [PubMed]
- Šeklić, D.S.; Jovanović, M.M.; Virijević, K.D.; Grujić, J.N.; Živanović, M.N.; Marković, S.D. Pseudevernia furfuracea inhibits migration and invasion of colorectal carcinoma cell lines. J. Ethnopharmacol. 2022, 284, 114758. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, H.; Lu, X.; Wen, C.; Huo, Z.; Shi, M.; Tang, X.; Chen, H.; Peng, C.; Fang, Y.; et al. Melittin-induced long non-coding RNA NONHSAT105177 inhibits proliferation and migration of pancreatic ductal adenocarcinoma. Cell Death Dis. 2018, 9, 940. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Lin, W.; Yin, Z.; Zou, Y.; Liang, S.; Ruan, S.; Chen, P.; Li, S.; Shu, Q.; Cheng, B.; et al. Melittin inhibits hypoxia-induced vasculogenic mimicry formation and epithelial-mesenchymal transition through suppression of HIF-1 α/Akt pathway in liver cancer. Evid.-Based Complement. Altern. Med. 2019, 2019, 9602935. [Google Scholar] [CrossRef] [PubMed]
- Di Zazzo, E.; Galasso, G.; Giovannelli, P.; Di Donato, M.; Bilancio, A.; Perillo, B.; Sinisi, A.A.; Migliaccio, A.; Castoria, G. Estrogen receptors in epithelial-mesenchymal transition of prostate cancer. Cancers 2019, 11, 1418. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.; DiLeo, A.; Niv, Y.; Gustafsson, J.Å. Estrogen receptor beta as target for colorectal cancer prevention. Cancer Lett. 2016, 372, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.M.; Isohama, Y.; Maruyama, H.; Yamada, Y.; Narita, Y.; Ohta, S.; Araki, Y.; Miyata, T.; Mishima, S. Estrogenic activities of fatty acids and a sterol isolated from royal jelly. Evid.-Based Complement. Altern. Med. 2008, 5, 295–302. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).