Antioxidant and Anti-Inflammatory Activities of Ethanol Extract from Daucus crinitus Desf. †
Abstract
1. Introduction
2. Results and Discussion
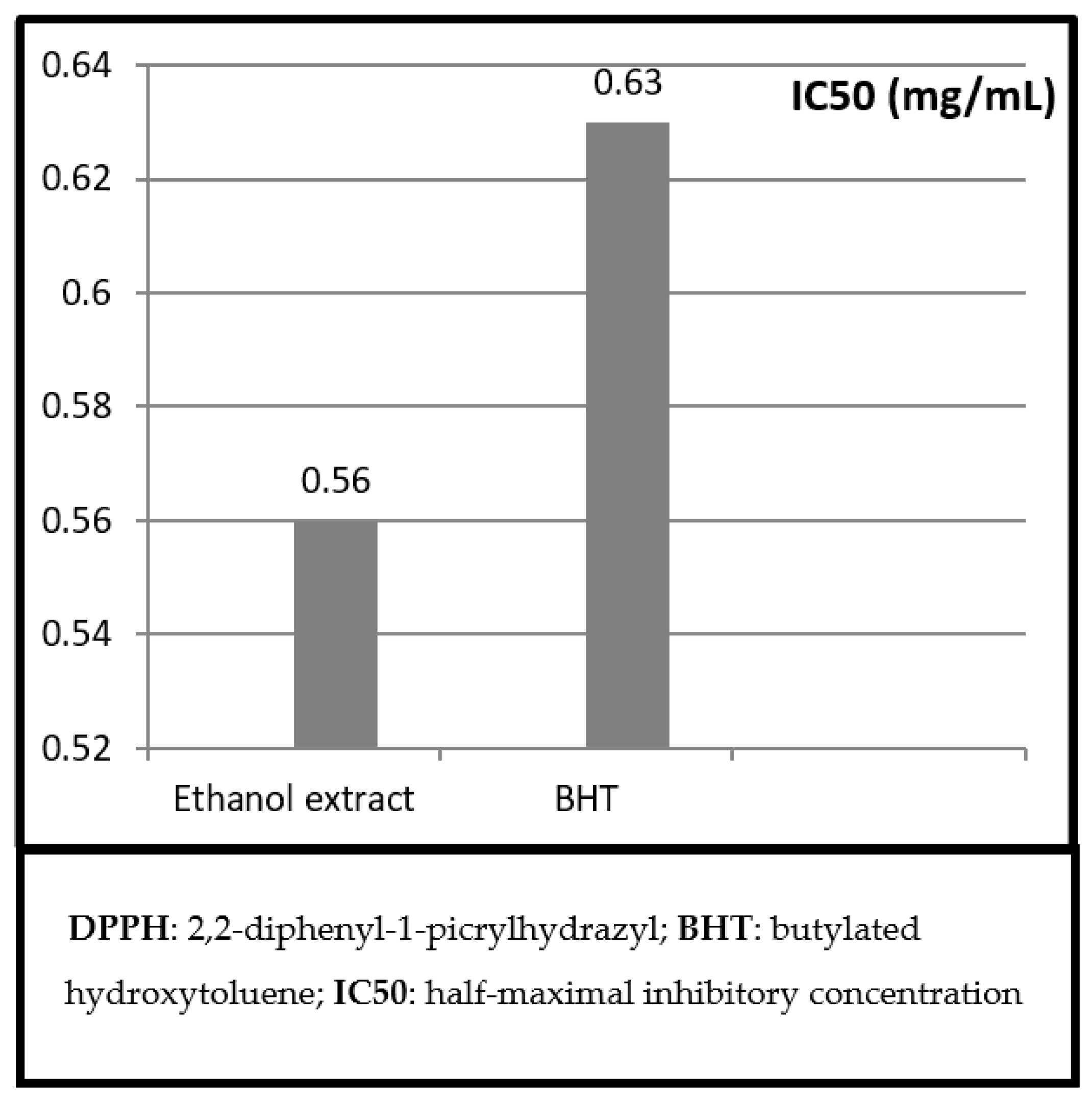
2.1. DPPH Free Radical Scavenging Assay
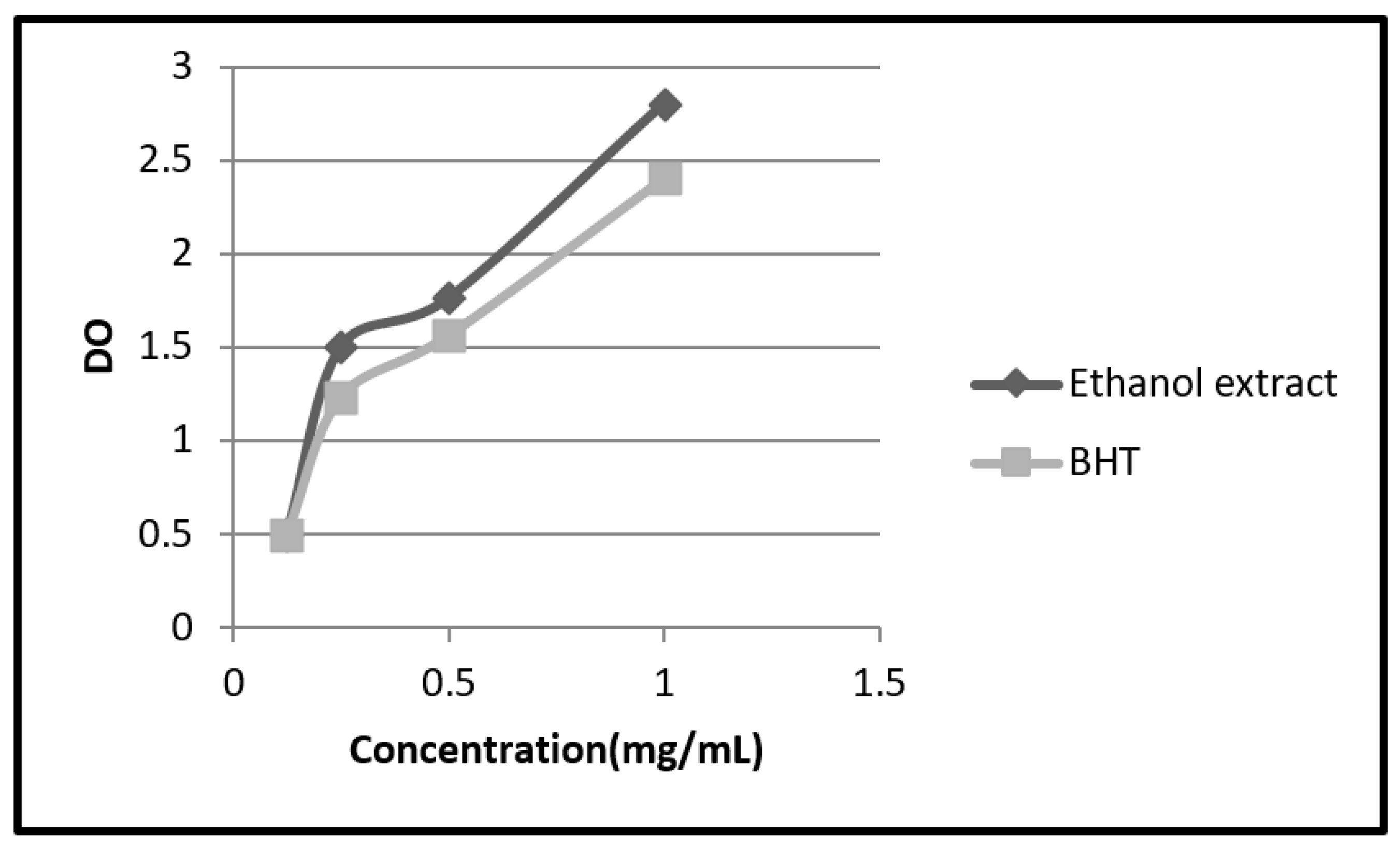
2.2. Ferric-Reducing Antioxidant Power Assay
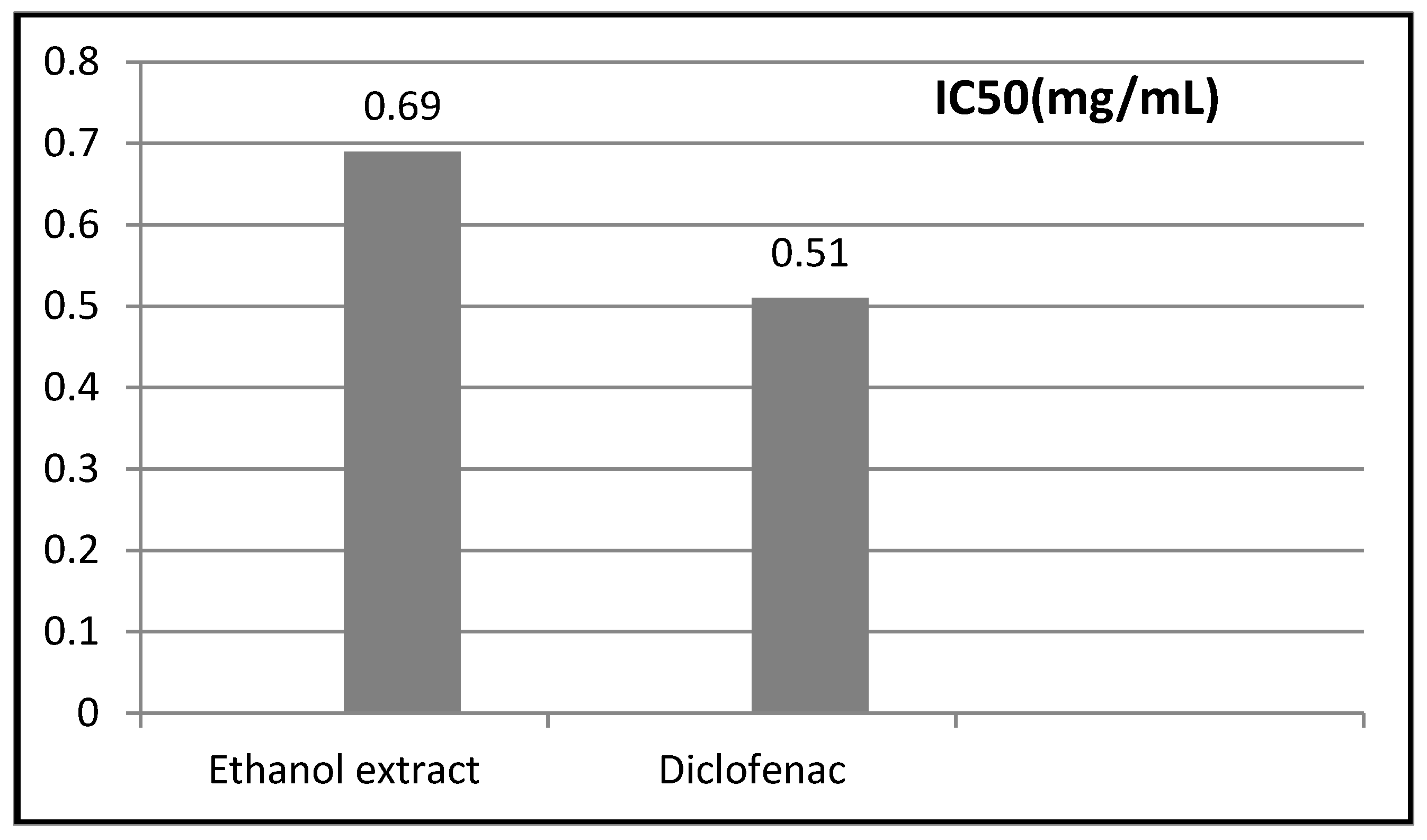
2.3. Anti-Inflammatory Activity
3. Materials and Methods
3.1. Plant Material and Extract Preparation
3.2. DPPH Free Radical Scavenging Assay
3.3. Ferric-Reducing Antioxidant Power Assay
3.4. Anti-Inflammatory Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Agraval, P.K. Carbon-13 NMR of Flavonoids. Studies in Organic Chemistry; Elsevier: Amsterdam, The Netherlands, 1989; Volume 39, p. 564. [Google Scholar]
- Lewis, K. Antibiotics: Recover the lost art of drug discovery. Nature 2012, 485, 439–440. [Google Scholar] [CrossRef]
- Javanmardi, J.; Stushnoff, C.; Locke, E.; Vivanco, J.M. Antioxidant activity and total phenolic content of Iranian Ocimum accessions. Food Chem. 2003, 83, 547–550. [Google Scholar] [CrossRef]
- Vilioglu, Y.S.; Mazza, G.; Gao, L.; Oomah, B.D. Antioxidant activity and total phenolics in selected fruits, vegetables and grain products. J. Agric. Food Chem. 1998, 46, 4113–4117. [Google Scholar] [CrossRef]
- Pietta, P.G. Flavonoids in Medicinal Plants. In Flavonoids in Health and Disease; Rice-Evans, C.A., Packer, L., Eds.; Dekker: New York, NY, USA, 1998; pp. 61–110. [Google Scholar]
- Shahidi, F.; Janitha, P.K.; Wanasundara, P.K.J.P.D. Phenolic antioxidants. Crit. Rev. Food. Sci. Nutr. 1992, 32, 67–103. [Google Scholar] [CrossRef]
- Sayed-Ahmad, B.; Talou, T.; Saad, Z.; Hijazi, A.; Merah, O. The Apiaceae: Ethnomedicinal family as a source for industrial uses. Ind. Crop. Prod. 2017, 109, 661–671. [Google Scholar] [CrossRef]
- El Amine Dib, M.; Djabou, N.; Desjobert, J.M.; Allali, H.; Tabti, B.; Muselli, A.; Costa, J. Characterization of volatile compounds of Daucus crinitus Desf.: Headspace Solid Phase Microextraction as an alternative technique to Hydrodistillation. Chem. Cent. J. 2010, 4, 16. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Quézel, P.; Santa, S. Nouvelle Flore de l’Algérie et des Régions Désertiques Méridionales; Éditions du Centre National de la Recherche Scientifique: Paris, France, 1962–1963. [Google Scholar]
- Cao, G.; Sofic, E.; Prior, R.L. Antioxidant Capacity of Tea and Common Vegetables. J. Agric. Food Chem. 1996, 44, 3426–3431. [Google Scholar] [CrossRef]
- Capecka, E.; Mareczek, A.; Lejas, M. Antioxidant activity of fresh and dry herbs of some Lamiaceae species. Food Chem. 2005, 93, 223–226. [Google Scholar] [CrossRef]
- Hatami, T.; Emami, S.A.; Miraghaee, S.S.; Mojarrab, M. Total phenolic contents and antioxidant activities of different extracts and fractions from the aerial parts of Artemisia biennis Willd. Iran. J. Pharm. Res. 2014, 13, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Al-Qudah, M.A.; Obeidat, S.M.; Saleh, A.M.; El-Oqlah, A.A.; Al-Masaeed, E.; Al-Jaber, H.I.; Abu Orabi, S.T. Volatile components analysis, total phenolic, flavonoid contents, and antioxidant activity of Phlomis species collected from Jordan. J. Essent. Oil Bear. Plants 2018, 21, 583–599. [Google Scholar] [CrossRef]
| Chemical Family | Flavonoids | Alkaloids | Tannins | Phenolic | Coumarins |
|---|---|---|---|---|---|
| Ethanol extract | + | − | + | + | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manel, F.; El Amine, D.M.; Okkacha, B. Antioxidant and Anti-Inflammatory Activities of Ethanol Extract from Daucus crinitus Desf. Chem. Proc. 2024, 16, 36. https://doi.org/10.3390/ecsoc-28-20164
Manel F, El Amine DM, Okkacha B. Antioxidant and Anti-Inflammatory Activities of Ethanol Extract from Daucus crinitus Desf. Chemistry Proceedings. 2024; 16(1):36. https://doi.org/10.3390/ecsoc-28-20164
Chicago/Turabian StyleManel, Fellahi, Dib Mohammed El Amine, and Bensaid Okkacha. 2024. "Antioxidant and Anti-Inflammatory Activities of Ethanol Extract from Daucus crinitus Desf." Chemistry Proceedings 16, no. 1: 36. https://doi.org/10.3390/ecsoc-28-20164
APA StyleManel, F., El Amine, D. M., & Okkacha, B. (2024). Antioxidant and Anti-Inflammatory Activities of Ethanol Extract from Daucus crinitus Desf. Chemistry Proceedings, 16(1), 36. https://doi.org/10.3390/ecsoc-28-20164






