The Effect of Curing Mode on the Parameters of Molecular Meshes of Epoxy and Polyester Copolymers †
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Method of Obtaining Samples
2.3. Methods of the Analysis of the Samples
3. Results and Discussion
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gandini, A.; Lacerda, T.M. From monomers to polymers from renewable resources: Recent advances. Prog. Polym. Sci. 2015, 48, 1–39. [Google Scholar] [CrossRef]
- Wang, G.; Jiang, M.; Zhang, Q.; Wang, R.; Zhou, G. Biobased copolyesters: Synthesis, crystallization behavior, thermal and mechanical properties of poly (ethylene glycol sebacate-co-ethylene glycol 2, 5-furan dicarboxylate). RSC Adv. 2017, 7, 13798–13807. [Google Scholar] [CrossRef]
- Pascault, J.-P.; Williams, R.J.J. (Eds.) Epoxy polymers. In New Materials and Innovations; Wiley VCH: New York, NY, USA, 2010. [Google Scholar]
- Irzhak, V.I.; Rosenberg, B.A.; Enikopyan, N.S. Cross-linked polymers. In Synthesis, Structure and Properties; Nauka: Moscow, Russia, 1979. (In Russian) [Google Scholar]
- Fink, J.K. Reactive polymers. Fundamentals and applications. In A Concise Guide to Industrial Polymers; William Andrew Publ.: Norwich, NY, USA, 2005; p. 139. [Google Scholar]
- Sorokin, M.F.; Kochnova, Z.A.; Shode, L.G. Chemistry and Technology of Film-Forming Agents; Khimia: Moscow, Russia, 1989; p. 476. (In Russian) [Google Scholar]
- Kerber, M.; Vinogradov, V.; Golovkin, G.; Gorbatkina, Y.; Kryzhanovsky, V.; Kuperman, A.; Simonov-Yemelyanov, I.; Khaliulin, V.; Bunakov, V. Polymer composite materials. In Properties, Structure, Technologies; Profession: Moscow, Russia, 2008; 560p. [Google Scholar]
- Dornbusch, M.; Christ, U.; Rasing, R. Epoxy Resins; Vincentz Network GmbH: Hannover, Germany, 2016. [Google Scholar]
- Vikhareva, I.N. The effect of the hardener on the characteristics of the polyester-based coating. Eng. Proc. 2024, 67, 16. [Google Scholar]
- Wang, R.; Wang, Z.G. Theory of polymer chains in poor solvent: Single-chain structure, solution thermodynamics, and θ point. Macromolecules 2014, 47, 4094–4102. [Google Scholar] [CrossRef]
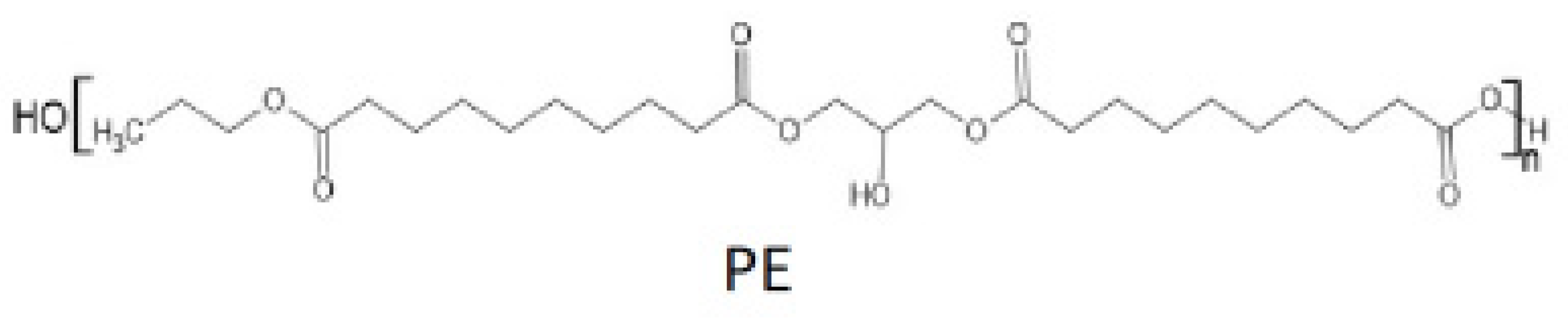
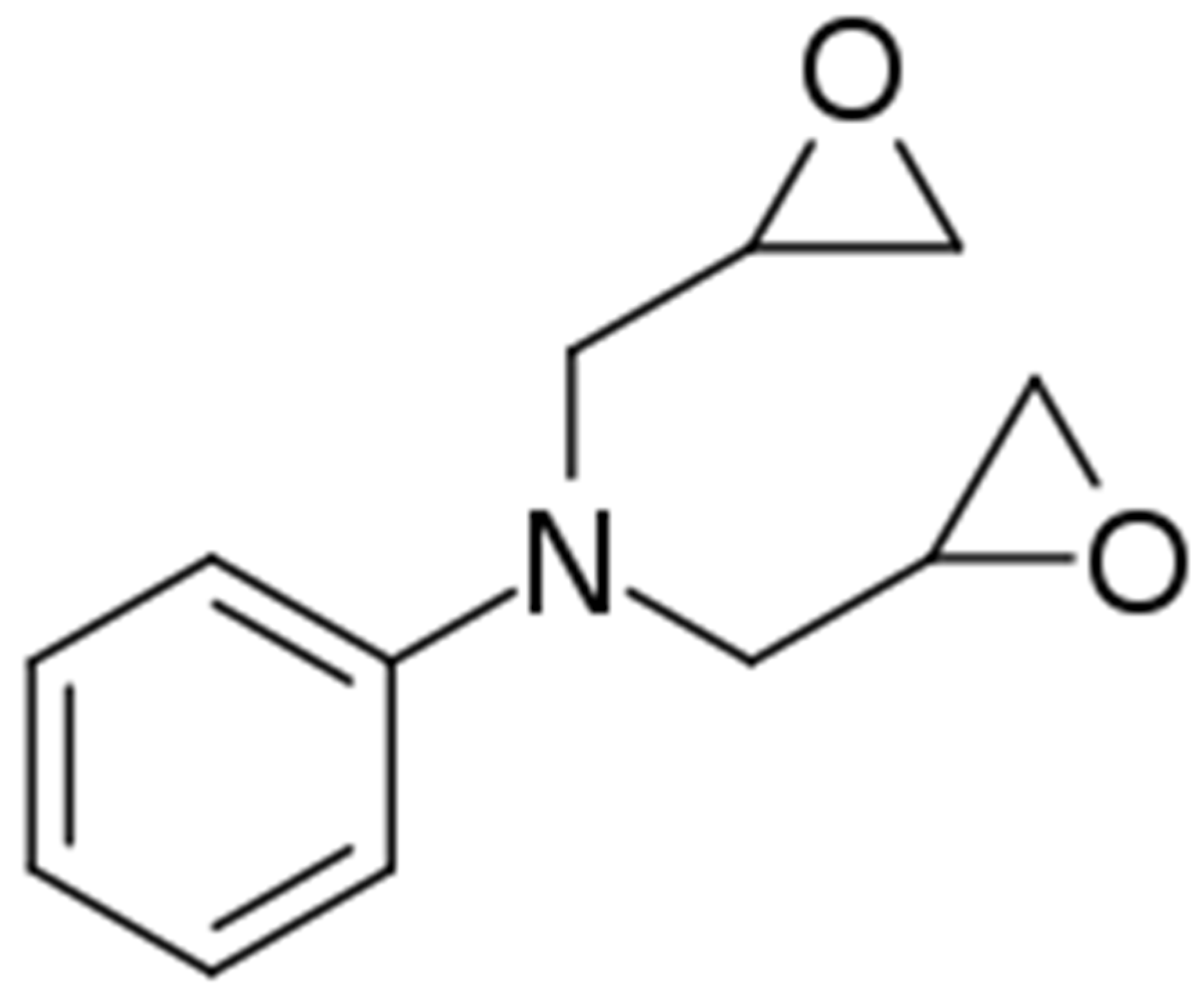
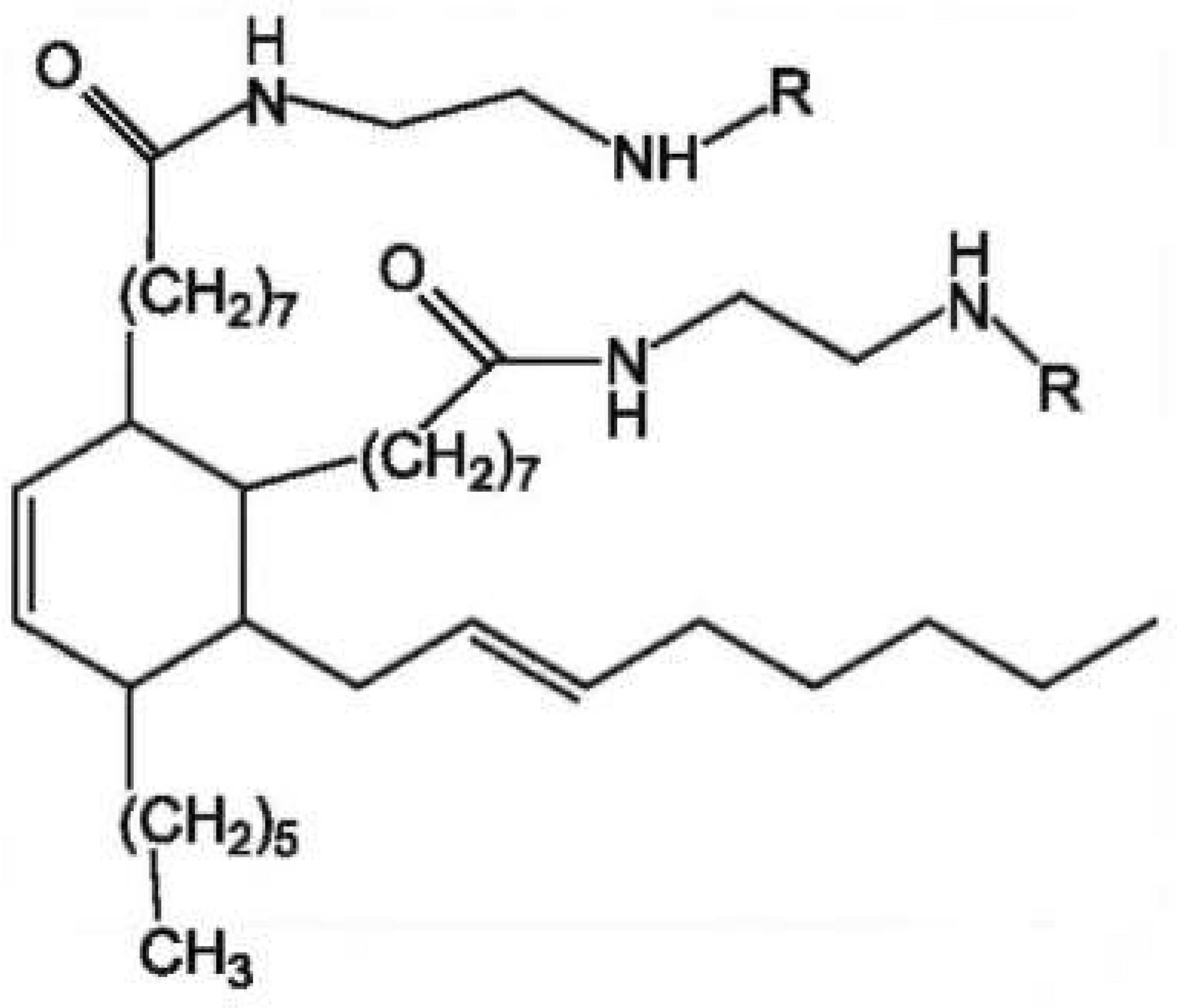
| Sample | Curing Modes |
|---|---|
| 1 | 22 °C/56 h |
| 2 | 22 °C/24 h, 60 °C/1 h, 80 °C/1 h |
| 3 | 22 °C/24 h, 80 °C/1 h, 100 °C/1 h, 120 °C/1 h |
| 4 | 60 °C/1 h, 80 °C/1 h, 120 °C/1 h |
| 5 | 22 °C/24 h, 80 °C/1 h, 120 °C/3 h |
| Sample | The Density Values, g/cm3 |
|---|---|
| 1 | 1.21 |
| 2 | 1.30 |
| 3 | 1.22 |
| 4 | 1.34 |
| 5 | 1.10 |
| Sample | S, % | j, % | Va, % | A | γ |
|---|---|---|---|---|---|
| 1 | 21.78 | 3.78 | 60.66 | 0.264 | 1.46 |
| 2 | 27.54 | 3.04 | 52.05 | 0.249 | 1.25 |
| 3 | 20.69 | 3.96 | 62.37 | 0.269 | 1.51 |
| 4 | 22.56 | 3.66 | 59.47 | 0.263 | 1.43 |
| 5 | 15.99 | 5.00 | 70.01 | 0.287 | 1.79 |
| Sample | Χ | |||
|---|---|---|---|---|
| Chloroform | Xylene | Dimethylformamide | Acetone | |
| 3 | 0.839 | 0.721 | 0.882 | 0.737 |
| Sample | Ds, % | Mc, g/mol | Nc × 1023, 1/cm3 | nc, mol/cm3 |
|---|---|---|---|---|
| 1 | 44.44 | 74.26 | 0.0980 | 0.016 |
| 2 | 43.09 | 67.67 | 0.1156 | 0.019 |
| 3 | 52.84 | 76.09 | 0.0965 | 0.016 |
| 4 | 67.98 | 79.69 | 0.1012 | 0.017 |
| 5 | 46.92 | 77.01 | 0.0860 | 0.014 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vikhareva, I.N. The Effect of Curing Mode on the Parameters of Molecular Meshes of Epoxy and Polyester Copolymers. Chem. Proc. 2024, 16, 34. https://doi.org/10.3390/ecsoc-28-20192
Vikhareva IN. The Effect of Curing Mode on the Parameters of Molecular Meshes of Epoxy and Polyester Copolymers. Chemistry Proceedings. 2024; 16(1):34. https://doi.org/10.3390/ecsoc-28-20192
Chicago/Turabian StyleVikhareva, Irina N. 2024. "The Effect of Curing Mode on the Parameters of Molecular Meshes of Epoxy and Polyester Copolymers" Chemistry Proceedings 16, no. 1: 34. https://doi.org/10.3390/ecsoc-28-20192
APA StyleVikhareva, I. N. (2024). The Effect of Curing Mode on the Parameters of Molecular Meshes of Epoxy and Polyester Copolymers. Chemistry Proceedings, 16(1), 34. https://doi.org/10.3390/ecsoc-28-20192





