New 1Z,5Z-Diene Compounds: Stereoselective Synthesis of Tetraenoic Macrodiolides †
Abstract
1. Introduction
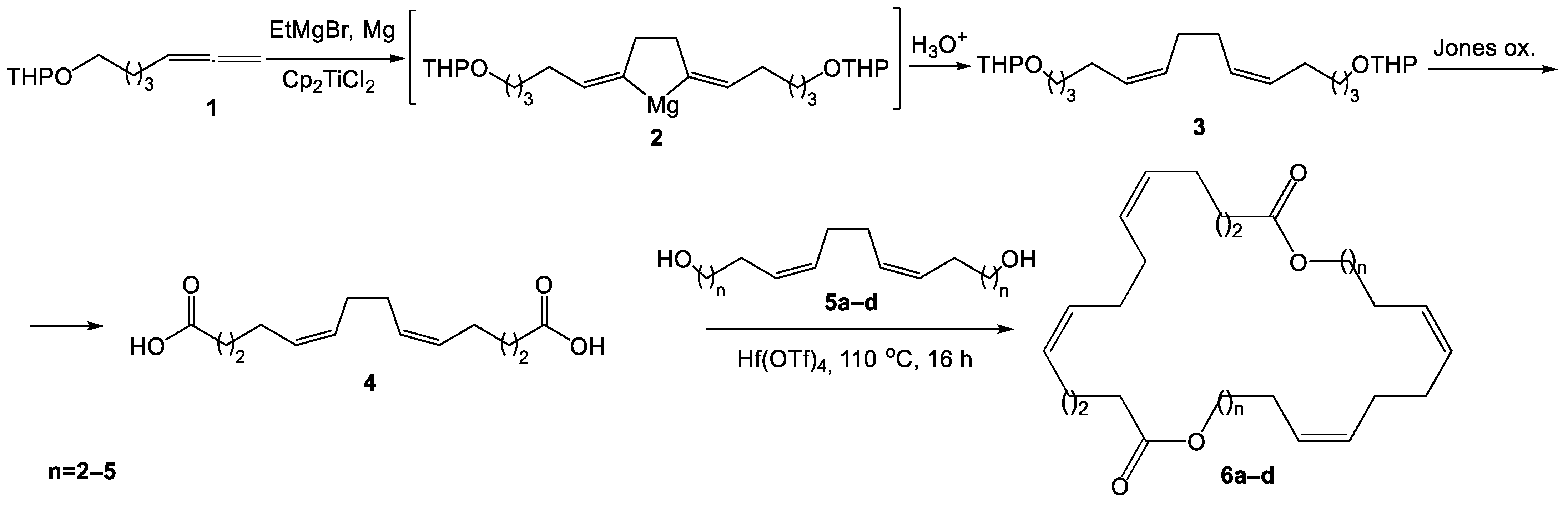
2. Results and Discussion
3. Materials and Methods
Chemistry
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Caffrey, P.; Hogan, M.; Song, Y. New Glycosylated Polyene Macrolides: Refining the Ore from Genome Mining. Antibiotics 2022, 11, 334. [Google Scholar] [CrossRef]
- Lenz, K.D.; Klosterman, K.E.; Mukundan, H.; Kubicek-Sutherland, J.Z. Macrolides: From Toxins to Therapeutics. Toxins 2021, 13, 347. [Google Scholar] [CrossRef] [PubMed]
- Carolus, H.; Pierson, S.; Lagrou, K.; Van Dijck, P. Amphotericin B and Other Polyenes—Discovery, Clinical Use, Mode of Action and Drug Resistance. J. Fungi 2020, 6, 321. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Jiang, H.; Liu, X.-W.; Zhou, J.; Wu, B. Polyene Macrolactams from Marine and Terrestrial Sources: Structure, Production Strategies, Biosynthesis and Bioactivities. Mar. Drugs 2022, 20, 360. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Dong, Y.; Zhou, H.; Cui, H. Effect of Post–Polyketide Synthase Modification Groups on Property and Activity of Polyene Macrolides. Antibiotics 2023, 12, 119. [Google Scholar] [CrossRef]
- Shurpik, D.N.; Akhmedov, A.A.; Cragg, P.J.; Plemenkov, V.V.; Stoikov, I.I. Progress in the Chemistry of Macrocyclic Meroterpenoids. Plants 2020, 9, 1582. [Google Scholar] [CrossRef] [PubMed]
- Caprioglio, D.; Salamone, S.; Pollastro, F.; Minassi, A. Biomimetic Approaches to the Synthesis of Natural Disesquiterpenoids: An Update. Plants 2021, 10, 677. [Google Scholar] [CrossRef] [PubMed]
- Olsufyeva, E.N.; Yankovskaya, V.S. Main trends in the design of semi-synthetic antibiotics of a new generation. Russ. Chem. Rev. 2020, 89, 339–378. [Google Scholar]
- Haro-Reyes, T.; Díaz-Peralta, L.; Galván-Hernández, A.; Rodríguez-López, A.; Rodríguez-Fragoso, L.; Ortega-Blake, I. Polyene Antibiotics Physical Chemistry and Their Effect on Lipid Membranes; Impacting Biological Processes and Medical Applications. Membranes 2022, 12, 681. [Google Scholar] [CrossRef] [PubMed]
- Dzhemileva, L.U.; D’yakonov, V.A.; Islamov, I.I.; Yunusbaeva, M.M.; Dzhemilev, U.M. New 1Z,5Z-diene macrodiolides: Catalytic synthesis, anticancer activity, induction of mitochondrial apoptosis, and effect on the cell cycle. Bioorg. Chem. 2020, 99, 103832. [Google Scholar] [CrossRef]
- D’yakonov, V.A.; Islamov, I.I.; Dzhemileva, L.U.; Makarova, E.K.; Dzhemilev, U.M. Direct synthesis of polyaromatic cyclophanes containing bis-methylene-interrupted Z-double bonds and study of their antitumor activity in vitro. Int. J. Mol. Sci. 2021, 22, 8787. [Google Scholar] [CrossRef] [PubMed]
- Islamov, I.I.; Dzhemileva, L.U.; Gaisin, I.V.; Dzhemilev, U.M.; D′ yakonov, V.A. New Polyether Macrocycles as Promising Antitumor Agents—Targeted Synthesis and Induction of Mitochondrial Apoptosis. ACS Omega 2024, 9, 19923–19931. [Google Scholar] [CrossRef] [PubMed]
- D’yakonov, V.A.; Makarov, A.A.; Dzhemileva, L.U.; Makarova, E.K.; Khusnutdinova, E.K.; Dzhemilev, U.M. The facile synthesis of the 5Z,9Z-dienoic acids and their topoisomerase I inhibitory activity. Chem. Commun. 2013, 49, 8401–8403. [Google Scholar] [CrossRef] [PubMed]
- D’yakonov, V.A.; Dzhemileva, L.U.; Makarov, A.A.; Mulyukova, A.R.; Baev, D.S.; Khusnutdinova, E.K.; Tolstikova, T.G.; Dzhemilev, U.M. nZ,(n + 4)Z-Dienoic fatty acids: A new method for the synthesis and inhibitory action on topoisomerase I and IIα. Med. Chem. Res. 2016, 25, 30. [Google Scholar] [CrossRef]
- D’yakonov, V.A.; Dzhemileva, L.U.; Dzhemilev, U.M. Natural compounds with bis-methylene-interrupted Z-double bonds: Plant sources, strategies of total synthesis, biological activity, and perspectives. Phytochem. Rev. 2021, 20, 325–342. [Google Scholar]
- D’yakonov, V.A.; Makarov, A.A.; Dzhemileva, L.U.; Ramazanov, I.R.; Makarova, E.K.; Dzhemilev, U.M. Natural Trienoic Acids as Anticancer Agents: First Stereoselective Synthesis, Cell Cycle Analysis, Induction of Apoptosis, Cell Signaling and Mitochondrial Targeting Studies. Cancers 2021, 13, 1808. [Google Scholar] [CrossRef]
- Islamov, I.I.; Yusupova, A.V.; D’yakonov, V.A.; Dzhemilev, U.M. Synthesis of polyether macrodiolides based on acetylenic derivatives of (5Z, 9Z)-tetradeca-5, 9-diene-1, 14-dioic acid. Russ. Chem. Bull. 2023, 72, 2473–2483. [Google Scholar] [CrossRef]
- Islamov, I.I.; Makarov, A.A.; Makarova, E.K.; Yusupova, A.V.; D’yakonov, V.A.; Dzhemilev, U.M. Synthesis of macrocyclic and linear compounds with 1 Z, 5 Z-diene and alkynylcarbinol fragments based on (5 Z, 9 Z)-tetradeca-5, 9-diene-1, 14-diol. Russ. Chem. Bull. 2023, 72, 925–931. [Google Scholar] [CrossRef]
- Islamov, I.I.; Gaisin, I.V.; Dzhemilev, U.M.; D’yakonov, V.A. Synthesis of macrocyclic mono-and diolides based on new ω-hydroxyalkadienoic acids with (Z, Z)-1, 5-diene moiety. Russ. Chem. Bull. 2024, 73, 1623–1630. [Google Scholar]
- D’yakonov, V.A.; Makarov, A.A.; Makarova, E.K.; Dzhemilev, U.M. Novel organomagnesium reagents in synthesis. Catalytic cyclomagnesiation of allenes in the synthesis of N-, O-, and Si-substituted 1Z, 5Z-dienes. Tetrahedron 2013, 69, 8516–8526. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Islamov, I.; Gaisin, I. New 1Z,5Z-Diene Compounds: Stereoselective Synthesis of Tetraenoic Macrodiolides. Chem. Proc. 2024, 16, 33. https://doi.org/10.3390/ecsoc-28-20110
Islamov I, Gaisin I. New 1Z,5Z-Diene Compounds: Stereoselective Synthesis of Tetraenoic Macrodiolides. Chemistry Proceedings. 2024; 16(1):33. https://doi.org/10.3390/ecsoc-28-20110
Chicago/Turabian StyleIslamov, Ilgiz, and Ilgam Gaisin. 2024. "New 1Z,5Z-Diene Compounds: Stereoselective Synthesis of Tetraenoic Macrodiolides" Chemistry Proceedings 16, no. 1: 33. https://doi.org/10.3390/ecsoc-28-20110
APA StyleIslamov, I., & Gaisin, I. (2024). New 1Z,5Z-Diene Compounds: Stereoselective Synthesis of Tetraenoic Macrodiolides. Chemistry Proceedings, 16(1), 33. https://doi.org/10.3390/ecsoc-28-20110





