Transamination of 3-[(Dimethylamino)methylidene]-5-arylfuran-2(3H)-thiones with the Participation of 1,2-Phenylenediamine †
Abstract
1. Introduction
2. Results and Discussion
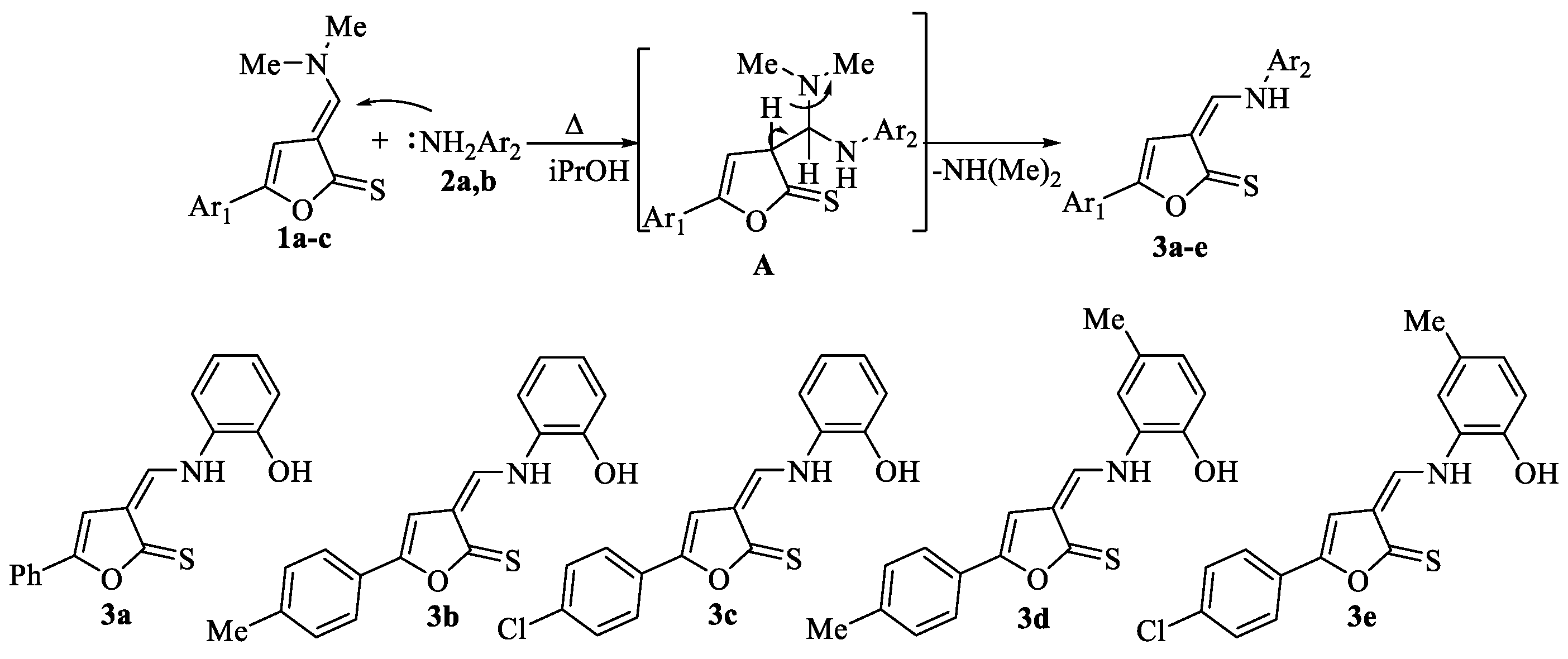
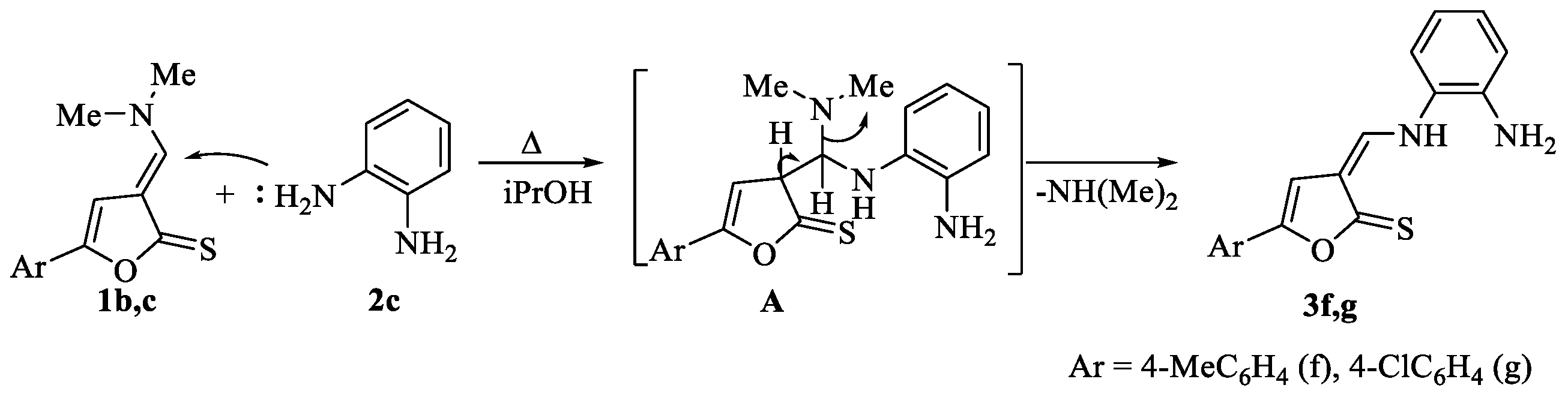
2.1. Synthesis of 3-[((2-Aminophenyl)amino)methylidene]-5-arylfuran-2(3H)-thiones
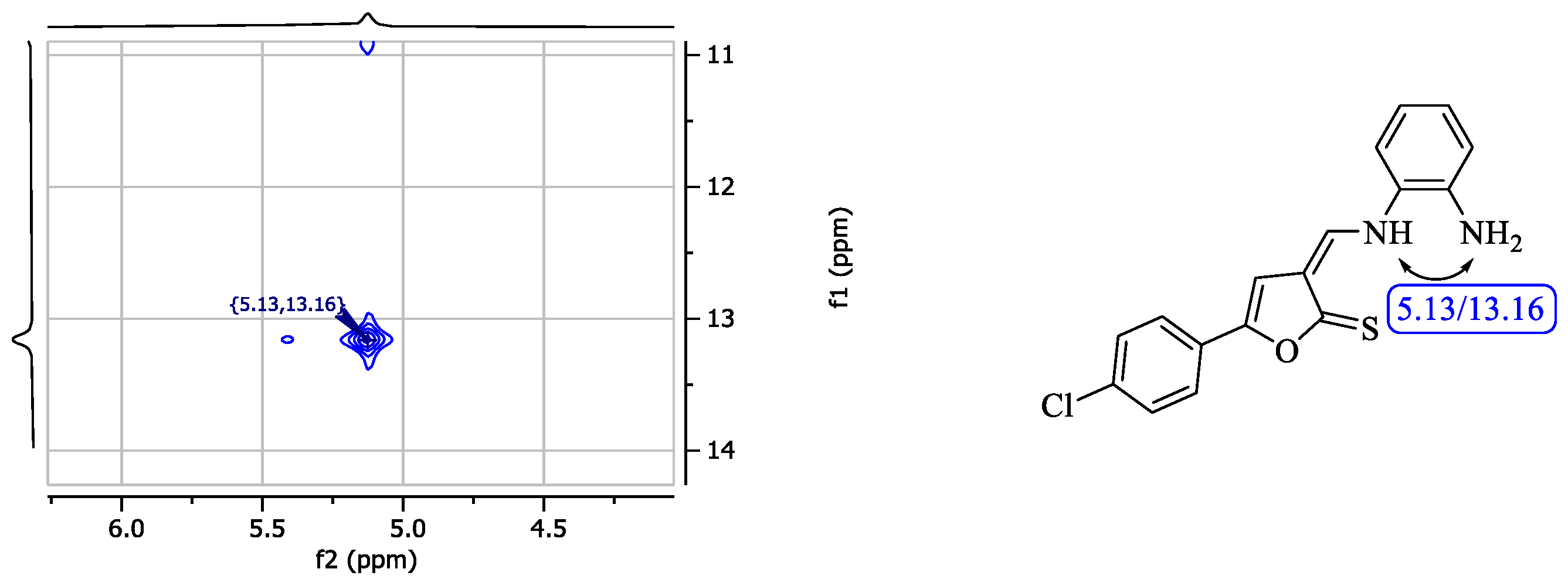
2.2. Structure of 3-[((2-Aminophenyl)amino)methylidene]-5-arylfuran-2(3H)-thiones 3f,g
3. Material and Methods
3.1. Physical Measurements
3.2. Synthesis and Characterization of Compounds 3f,g
- (Z)-3-[((2-aminophenyl)amino)methylidene]-5-(p-tolyl)furan-2(3H)-thione 3f
- (Z)-3-[((2-aminophenyl)amino)methylidene]-5-(4-chlorophenyl)furan-2(3H)-thione 3g
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Makawana, J.A.; Patel, M.P.; Patel, R.G. Synthesis and in vitro antimicrobial activity of N-arylquinoline derivatives bearing 2-morpholinoquinoline moiety. Chin. Chem. Lett. 2012, 23, 427–430. [Google Scholar] [CrossRef]
- Hundsdörfer, C.; Hemmerling, H.-J.; Götz, C.; Totzke, F.; Bednarski, P.; Le Borgne, M.; Jose, J. Indeno[1,2-b]indole derivatives as a novel class of potent human protein kinase CK2 inhibitors. Bioorg. Med. Chem. 2012, 20, 2282–2289. [Google Scholar] [CrossRef]
- Gonçalves, D.S.; Silva, M.J.V.; Souza, T.F.; Jacomini, A.P.; Back, D.F.; Basso, E.A.; Moura, S. Synthesis of a New Polyfunctionalised Pyrimidine-4-carboxylate and Its Application for the Construction of a Series of Pyrimidine Derivatives. Synthesis 2016, 48, 3042–3049. [Google Scholar] [CrossRef]
- Andrade, V.; Mittersteiner, M.; Bonacorso, H.; Frizzo, C.; Martins, M.; Zanatta, N. Regioselective Synthesis of 5-(Trifluoromethyl)[1,2,4]triazolo[1,5-a]pyrimidines from β-Enamino Diketones. Synthesis 2019, 51, 2311–2317. [Google Scholar] [CrossRef]
- Ali, M.; Barakat, A.; El-Faham, A.; Al-Majid, A.M.; Yousuf, S.; Ashraf, S.; Albericio, F. Enamine Barbiturates and Thiobarbiturates as a New Class of Bacterial Urease Inhibitors. Appl. Sci. 2020, 10, 3523–3535. [Google Scholar] [CrossRef]
- Kaping, S.; Kalita, U.; Sunn, M.; Singha, L.O.; Vishwakarma, J.N. A facile, regioselective synthesis of pyrazolo[1, 5-a]pyrimidine analogs in the presence of KHSO4 in aqueous media assisted by ultrasound and their anti-inflammatory and anti-cancer activities. Monatsh. Chem. 2016, 147, 1257–1276. [Google Scholar] [CrossRef]
- Abu-Bakr, S.M.; Khidre, M.D.; Omar, M.A.; Swelam, S.A.; Awad, H.M. Synthesis of furo[3,2-g]chromones under microwave irradiation and their antitumor activity evaluation. J. Heterocycl. Chem. 2019, 57, 731–743. [Google Scholar] [CrossRef]
- Tikhomolova, A.S.; Grinev, V.S.; Yegorova, A.Y. One-Pot Synthesis, E-/Z-Equilibrium in Solution of 3-Hetarylaminomethylidenefuran-2(3H)-ones and the Way to Selective Synthesis of the E-Enamines. Molecules 2023, 28, 963. [Google Scholar] [CrossRef]
- Tikhomolova, A.S.; Mamleeva, Z.V.; Yegorova, A.Y. An efficient synthesis of (E)-3-[(dimethylamino)methylidene]furan-2(3H)-thiones and transamination reactions thereof. Chem. Heterocycl. Compd. 2024, 60, 138–142. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tikhomolova, A.S.; Yegorova, A.Y. Transamination of 3-[(Dimethylamino)methylidene]-5-arylfuran-2(3H)-thiones with the Participation of 1,2-Phenylenediamine. Chem. Proc. 2024, 16, 31. https://doi.org/10.3390/ecsoc-28-20121
Tikhomolova AS, Yegorova AY. Transamination of 3-[(Dimethylamino)methylidene]-5-arylfuran-2(3H)-thiones with the Participation of 1,2-Phenylenediamine. Chemistry Proceedings. 2024; 16(1):31. https://doi.org/10.3390/ecsoc-28-20121
Chicago/Turabian StyleTikhomolova, Alexandra S., and Alevtina Yu. Yegorova. 2024. "Transamination of 3-[(Dimethylamino)methylidene]-5-arylfuran-2(3H)-thiones with the Participation of 1,2-Phenylenediamine" Chemistry Proceedings 16, no. 1: 31. https://doi.org/10.3390/ecsoc-28-20121
APA StyleTikhomolova, A. S., & Yegorova, A. Y. (2024). Transamination of 3-[(Dimethylamino)methylidene]-5-arylfuran-2(3H)-thiones with the Participation of 1,2-Phenylenediamine. Chemistry Proceedings, 16(1), 31. https://doi.org/10.3390/ecsoc-28-20121




