A Straightforward and Efficient Approach to the Synthesis of 3-Cyano-Coumarine Derivatives †
Abstract
1. Introduction
2. Results and Discussion
2.1. Alkenes Synthesis 3a–e
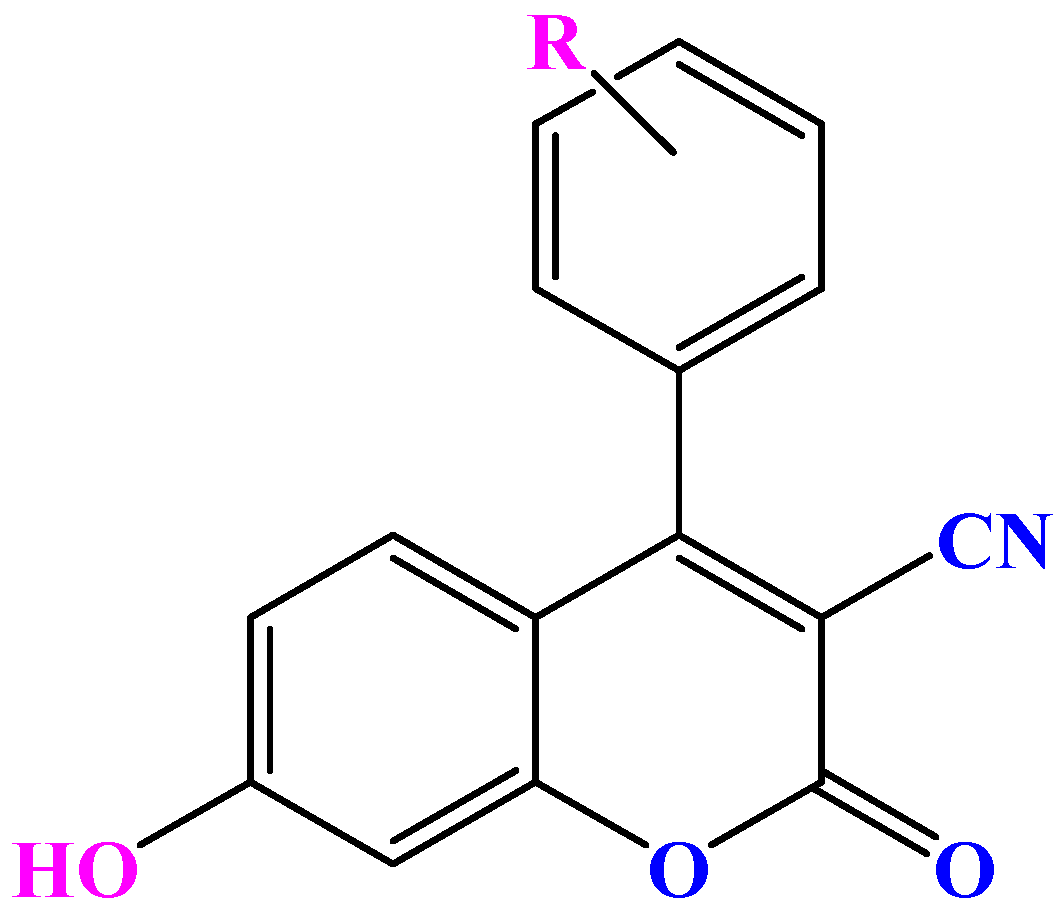
2.2. Synthesis of 3-Cyano-Coumarin Derivatives 5a–e
3. Experimental Procedures
3.1. General Synthesis of Alkyne Derivatives 3a–e
3.2. General Synthesis of 3-Cyano-Coumarin Derivatives 5a–e
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arora, P.; Arora, V.; Lamba, H.; Wadhwa, D. Importance of heterocyclic chemistry: A review. Int. J. Pharm. Sci. Res. 2012, 3, 2947. [Google Scholar]
- Bhattacherjee, D.; Zyryanov, G.V.; Das, P. Recent advances in the synthetic approaches to 2-pyridones (microreview). Chem. Heterocycl. Compd. 2020, 56, 1152–1154. [Google Scholar] [CrossRef]
- Kabir, E.; Uzzaman, M. A review on biological and medicinal impact of heterocyclic compounds. Results Chem 2022, 4, 100606. [Google Scholar] [CrossRef]
- Asmaa, K.; Fatima, B.; Zahira, K.; Noureddine, C.-B. Ten Years of Progress in the Synthesis of 2-Pyridone Derivatives via Three/Four Component Reaction. Mini-Rev. Org. Chem. 2023, 20, 358–371. [Google Scholar] [CrossRef]
- Patrusheva, O.S.; Volcho, K.P.; Salakhutdinov, N.F. Synthesis of oxygen-containing heterocyclic compounds based on monoterpenoids. Russ. Chem. Rev. 2018, 87, 771. [Google Scholar] [CrossRef]
- Cafeo, G.; Satira, A.; Russo, M.; Mondello, M.; Dugo, P. Determination of Oxygen Heterocyclic Compounds in Foods Using Supercritical Fluid Chromatography–Tandem Mass Spectrometry. Foods 2023, 12, 3408. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, B.; Ranga Rao, G. PWA/montmorillonite K10 catalyst for synthesis of coumarins under solvent-free conditions. J. Porous Mater. 2012, 19, 233–242. [Google Scholar] [CrossRef]
- Abdou, M.M. 3-Acetyl-4-hydroxycoumarin: Synthesis, reactions and applications. Arab. J. Chem. 2017, 10, S3664–S3675. [Google Scholar] [CrossRef]
- Slimani, I.; Hamzaoui, S.; Mansour, L.; Harrath, A.H.; Hamdi, N. One-pot, simple and efficient synthesis of novel bioactive 4-aryl-1, 2-dihydro-6-(4-hydroxy-2-oxo-2H-chromen-3-yl)-2-oxopyridin-3-carbonitriles via multi-component approach. J. King Saud Univ. Sci. 2020, 32, 1212–1217. [Google Scholar] [CrossRef]
- Siziani, D.; Ziani, B.E.C.; Abdi, Y.; Bensouilah, N.; Boutemeur-Kheddis, B.; Ziani, C.; Boukkena, L.; Hamdi, M.; Talhi, O.; Bachari, K. Multicomponent synthesis of pyranonicotinonitrile and chromene-3-carbonitrile: Studies on bioactivities and molecular docking. J. Mol. Struct. 2022, 1264, 13236. [Google Scholar] [CrossRef]
- Abu El-Azm, F.S.; El-Shahawi, M.M.; Elgubbi, A.S.; Madkour, H.M. Synthesis of new benzo [f] chromene-based heterocycles targeting anti-proliferative activity. J. Iran. Chem. Soc. 2021, 18, 1081–1092. [Google Scholar] [CrossRef]
- Zeydi, M.M.; Kalantarian, S.J.; Kazeminejad, Z. Overview on developed synthesis procedures of coumarin heterocycles. J. Iran. Chem. Soc. 2020, 17, 3031–3094. [Google Scholar] [CrossRef]
- Rezayati, S.; Sheikholeslami-Farahani, F.; Rostami-Charati, F.; Abad, S.A.S. One-pot synthesis of coumarine derivatives using butylenebispyridinium hydrogen sulfate as novel ionic liquid catalyst. Res. Chem. Intermed. 2016, 42, 4097–4107. [Google Scholar] [CrossRef]
- Bardasov, I.; Alekseeva, A.Y.; Malyshkina, N.; Ershov, O.; Surazhskaya, M.; Grishanov, D. New synthesis of 4-alkyl-3-cyanocoumarins. Russ. J. Org. Chem. 2016, 52, 983–986. [Google Scholar] [CrossRef]
- Molnar, M.; Lončarić, M.; Kovač, M. Green chemistry approaches to the synthesis of coumarin derivatives. Curr. Org. Chem. 2020, 24, 4–43. [Google Scholar] [CrossRef]
- Bardasov, I.; Malyshkina, N.; Alekseeva, A.Y.; Ershov, O.; Timrukova, D.; Grigor’eva, A. Synthesis and optical properties of new coumarin derivatives based on 2-(2-chlorobenzylidene) malononitrile. Russ. J. Org. Chem. 2017, 53, 47–50. [Google Scholar] [CrossRef]
 | ||
| Entry | R | Yield (%) |
| 3a | H | 33 |
| 3b | 4-Cl | 73 |
| 3c | 4-F | 79 |
| 3d | 3-CH3 | 59 |
| 3e | 3,4,5 tri-OMe | 90 |
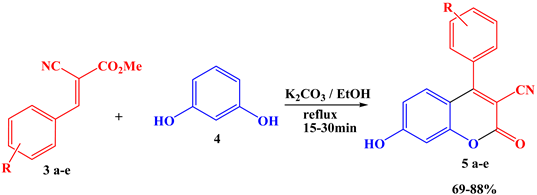 | ||
| Entry | R | Yield (%) |
| 3a | H | 71 |
| 3b | 4-Cl | 88 |
| 3c | 4-F | 69 |
| 3d | 3-CH3 | 73 |
| 3e | 3,4,5 tri-OMe | 77 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kebaili, A.; Belhadj, F.; Kibou, Z.; Seijas, J.A.; Tato, M.P.V.; Choukchou-Braham, N. A Straightforward and Efficient Approach to the Synthesis of 3-Cyano-Coumarine Derivatives. Chem. Proc. 2024, 16, 41. https://doi.org/10.3390/ecsoc-28-20113
Kebaili A, Belhadj F, Kibou Z, Seijas JA, Tato MPV, Choukchou-Braham N. A Straightforward and Efficient Approach to the Synthesis of 3-Cyano-Coumarine Derivatives. Chemistry Proceedings. 2024; 16(1):41. https://doi.org/10.3390/ecsoc-28-20113
Chicago/Turabian StyleKebaili, Asmaa, Fatima Belhadj, Zahira Kibou, Julio A. Seijas, M. Pilar Vazquez Tato, and Noureddine Choukchou-Braham. 2024. "A Straightforward and Efficient Approach to the Synthesis of 3-Cyano-Coumarine Derivatives" Chemistry Proceedings 16, no. 1: 41. https://doi.org/10.3390/ecsoc-28-20113
APA StyleKebaili, A., Belhadj, F., Kibou, Z., Seijas, J. A., Tato, M. P. V., & Choukchou-Braham, N. (2024). A Straightforward and Efficient Approach to the Synthesis of 3-Cyano-Coumarine Derivatives. Chemistry Proceedings, 16(1), 41. https://doi.org/10.3390/ecsoc-28-20113






