Microwave-Assisted Green Synthesis of Binary/Ternary ZnxCo1−xFe2O4 (x = 0, 0.5, 1) Nanoparticles †
Abstract
1. Introduction
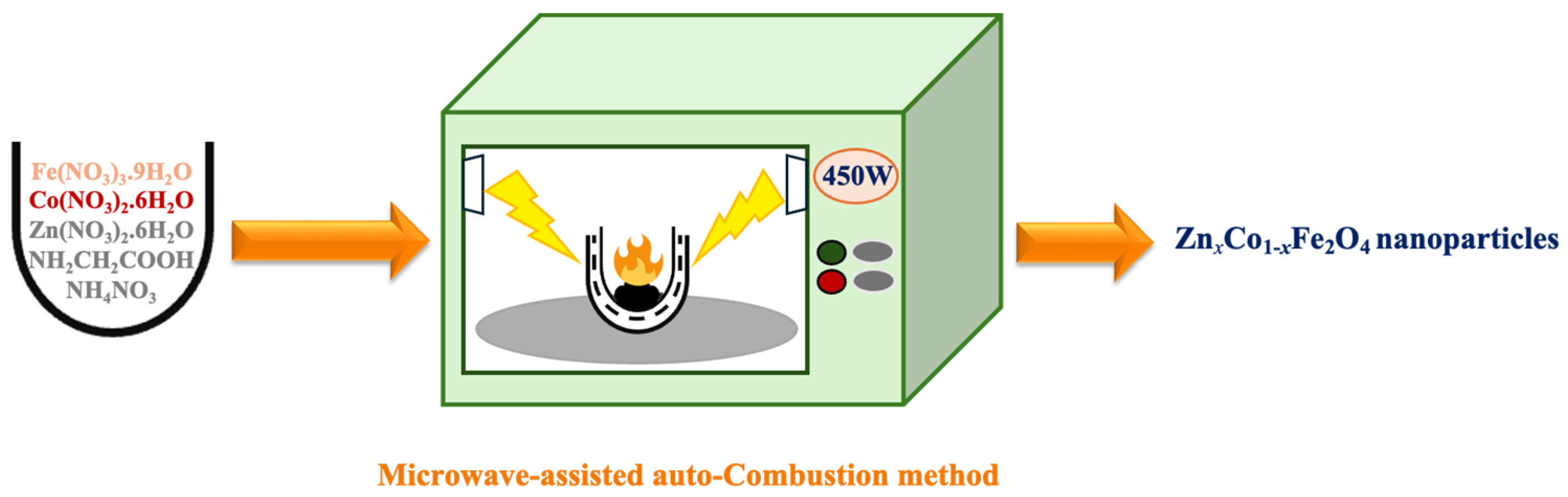
2. Experimental Section
- Synthesis of binary/ternary ZnxCo1−xFe2O4 (x = 0, 0.5, 1) nanoparticles:
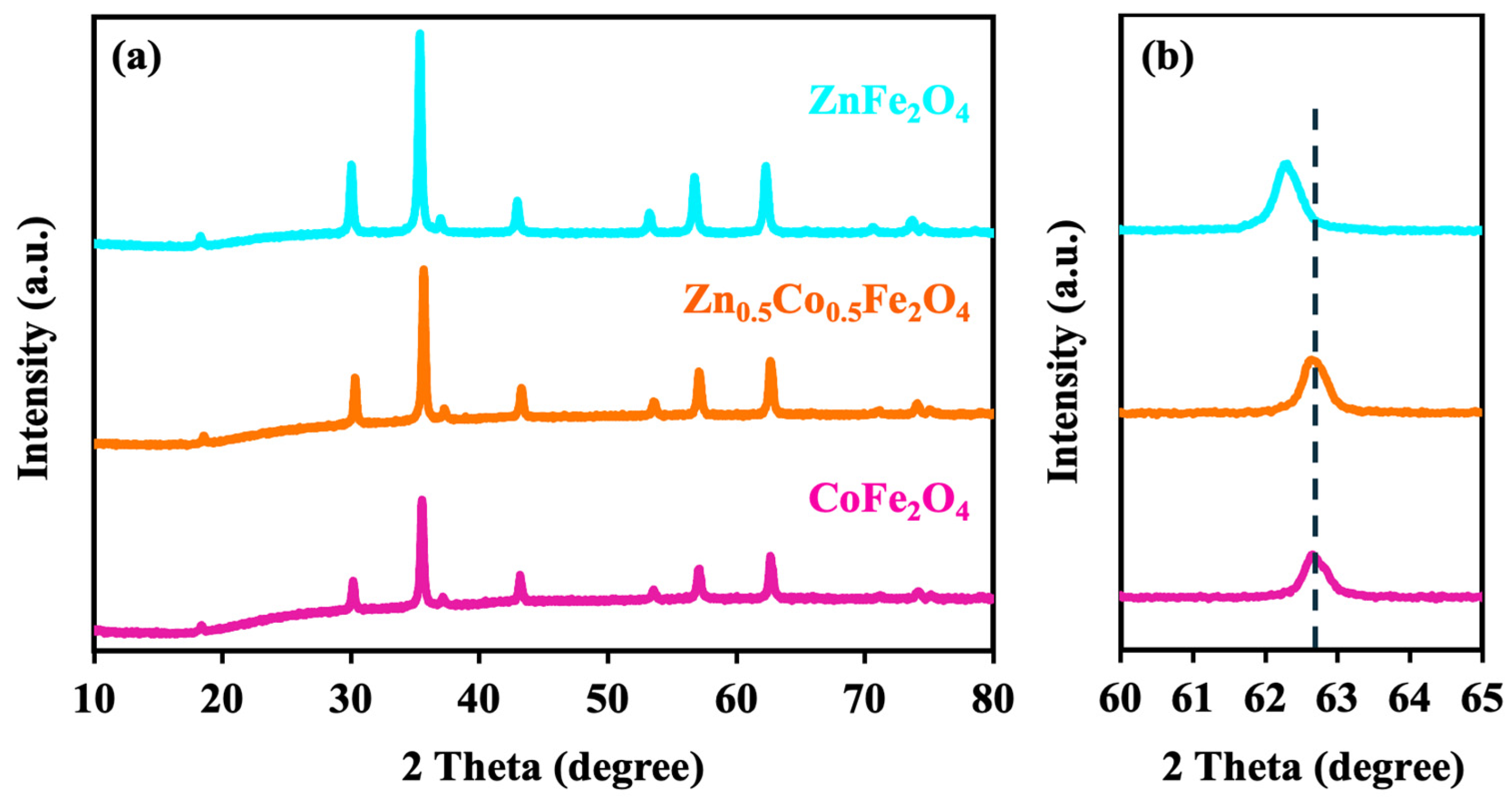
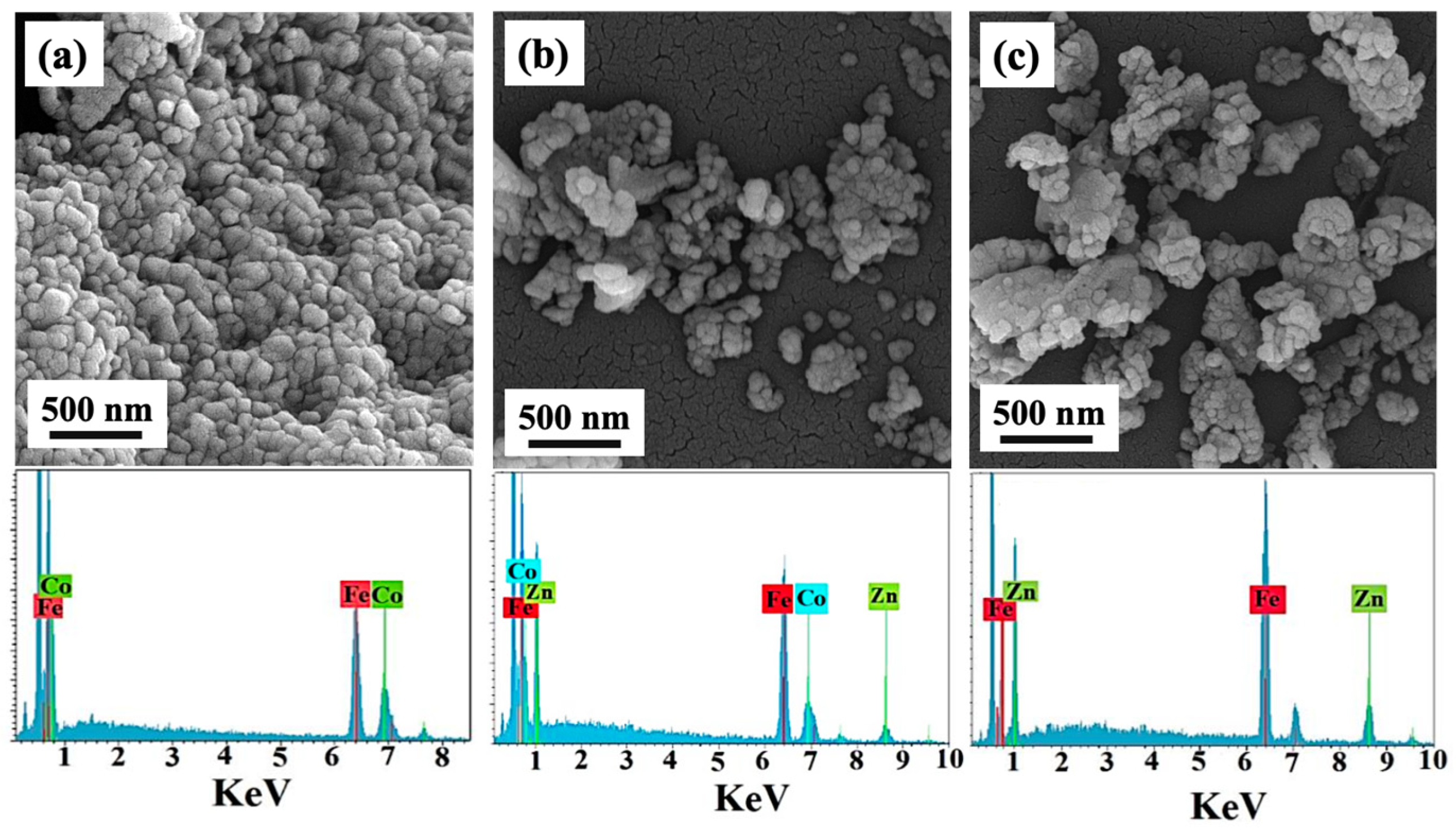
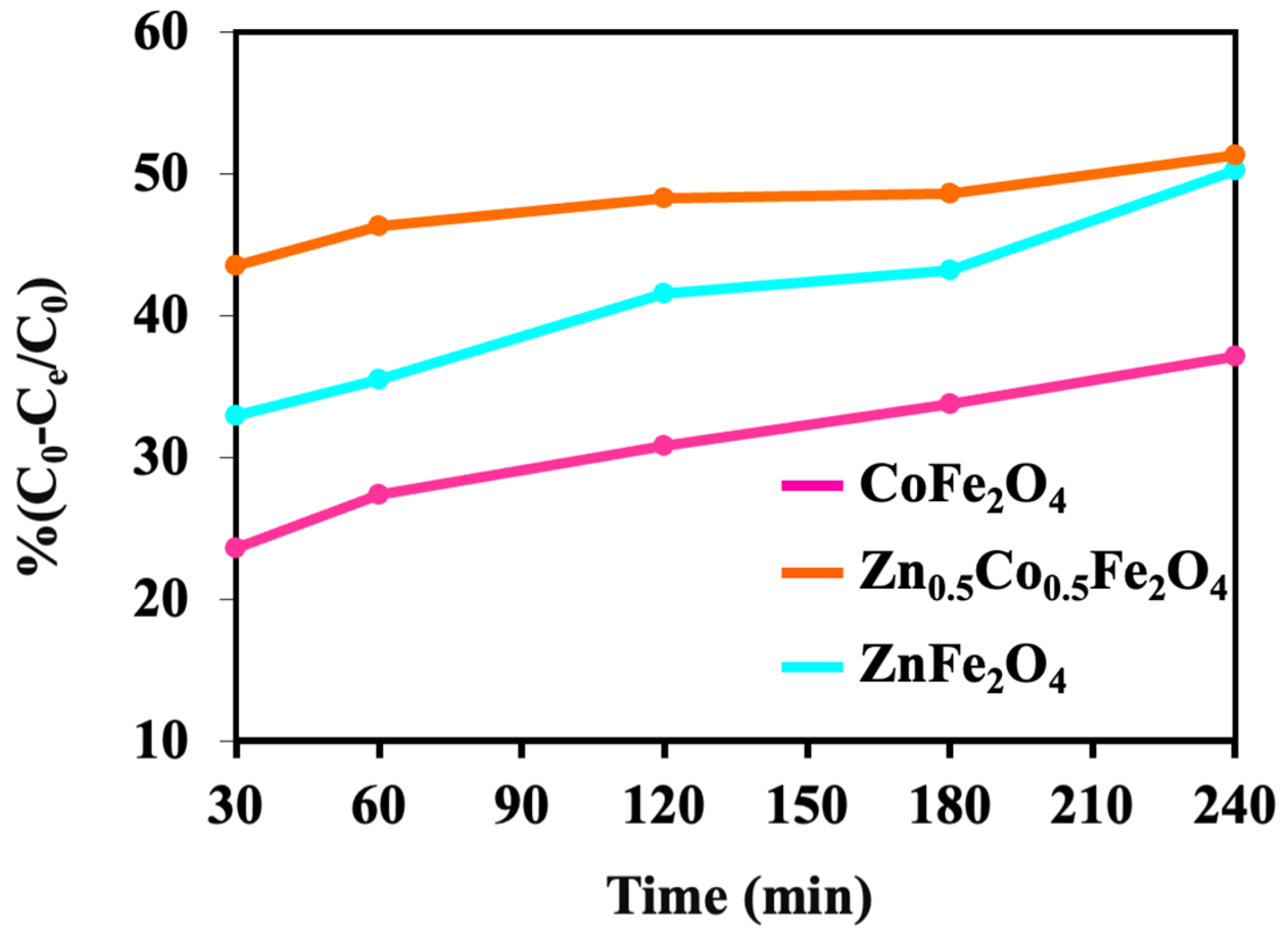
3. Result and Discussion
4. Photocatalytic Activity
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chahar, D.; Taneja, S.; Bisht, S.; Kesarwani, S.; Thakur, P.; Thakur, A.; Sharma, P.B. Photocatalytic activity of cobalt substituted zinc ferrite for the degradation of methylene blue dye under visible light irradiation. J. Alloys Compd. 2021, 851, 156878. [Google Scholar] [CrossRef]
- Kamel Attar Kar, M.; Fazaeli, R.; Manteghi, F.; Ghahari, M. Structural, Optical, and Isothermic Studies of CuFe2O4 and Zn-Doped CuFe2O4 Nanoferrite as a Magnetic Catalyst for Photocatalytic Degradation of Direct Red 264 Under Visible Light Irradiation. Environ. Prog. Sustain. Energy 2019, 38, 13109. [Google Scholar] [CrossRef]
- Swathi, S.; Yuvakkumar, R.; Kumar, P.S.; Ravi, G.; Velauthapillai, D. Annealing temperature effect on cobalt ferrite nanoparticles for photocatalytic degradation. Chemosphere 2021, 281, 130903. [Google Scholar] [CrossRef] [PubMed]
- Magdalane, C.M.; Priyadharsini, G.M.A.; Kaviyarasu, K.; Jothi, A.I.; Simiyon, G.G. Synthesis and characterization of TiO2 doped cobalt ferrite nanoparticles via microwave method: Investigation of photocatalytic performance of congo red degradation dye. Surf. Interfaces 2021, 25, 101296. [Google Scholar] [CrossRef]
- Alshammari, A.H.; Alshammari, K.; Alhassan, S.; Alshammari, M.; Alotaibi, T.; Alanzy, A.O.; Taha, T.A.M. Low temperature sol-gel synthesis of copper zinc ferrite for hydrogen catalytic hydrolysis of sodium borohydride. Mater. Chem. Phys. 2023, 308, 128287. [Google Scholar] [CrossRef]
- Bashar, A.; Molla, T.H.; Chandra, D.; Malitha, D.; Islam, S.; Rahman, S.; Ahsan, S. Hydrothermal synthesis of cobalt substitute zinc-ferrite (Co1−xZnxFe2O4) nanodot, functionalised by polyaniline with enhanced photocatalytic activity under visible light irradiation. Heliyon 2023, 9, 1538. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N.; Katoch, A.; Singh, S.; Kaur, R. Properties of zirconium ferrite nanoparticles prepared by hydrothermal process. Mater. Lett. 2023, 330, 133236. [Google Scholar] [CrossRef]
- Chamani, S.; Khatamian, M.; Peighambardoust, N.S.; Aydemir, U. Microwave-Assisted Auto-Combustion Synthesis of Binary/Ternary Cox Ni1−x Ferrite for Electrochemical Hydrogen and Oxygen Evolution. ACS Omega 2021, 6, 33024–33032. [Google Scholar] [CrossRef] [PubMed]
- Chamani, S.; Sadeghi, E.; Peighambardoust, N.S.; Doganay, F.; Yanalak, G.; Eroglu, Z.; Aslan, E.; Asghari, E.; Metin, O.; Patir, I.H.; et al. Photocatalytic hydrogen evolution performance of metal ferrites/polypyrrole nanocomposites. Int. J. Hydrogen Energy 2022, 47, 32940–32954. [Google Scholar] [CrossRef]
- Charles Prabakar, A.; Sathyaseelan, B.; Killivalavan, G.; Iruson, B.; Senthilnathan, K.; Manikandan, E.; Sivakumar, D. Photocatalytic dye degradation properties of zinc copper ferrites nanoparticles. J. Nanostruct. 2019, 9, 694–701. [Google Scholar]
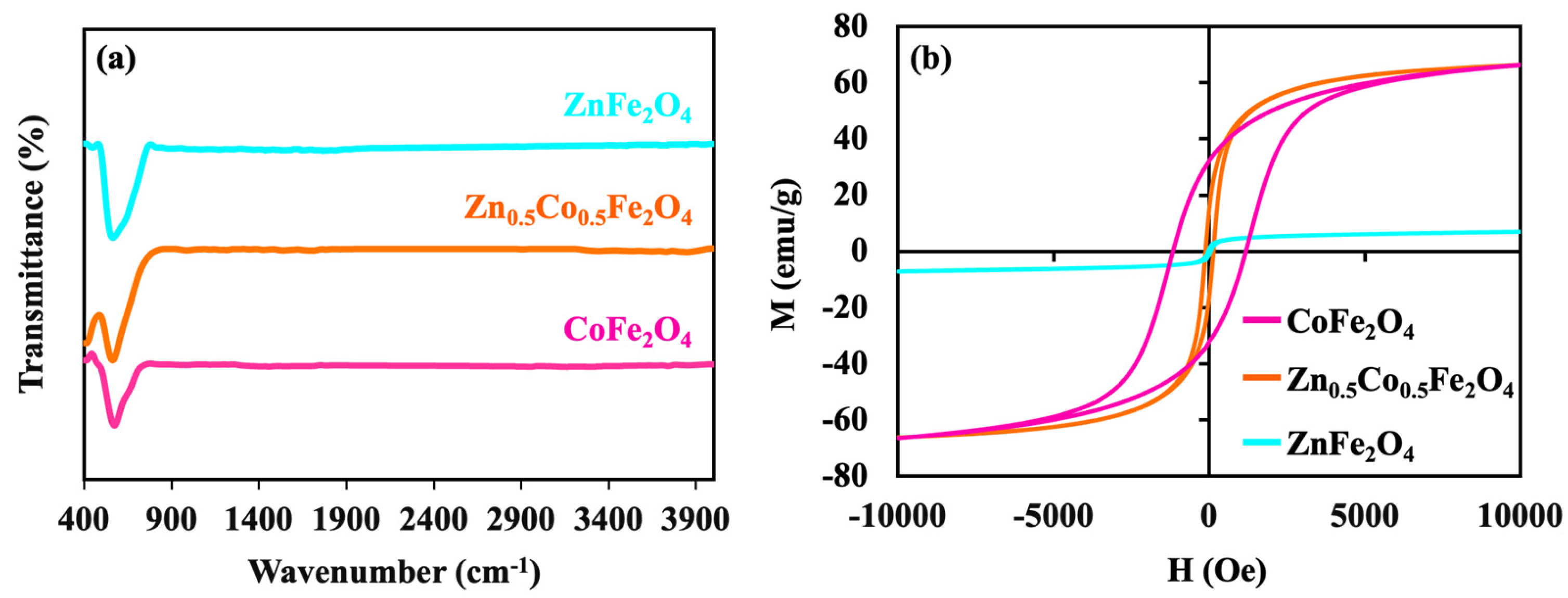
| Sample | Ms | Hc | Mr |
|---|---|---|---|
| CoFe2O4 | 66.4 | −1174.5 | 32.5 |
| Zn0.5Co0.5Fe2O4 | 66.3 | −128.4 | 16 |
| ZnFe2O4 | 7 | 47.5 | 1.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chamani, S.; Khatamian, M. Microwave-Assisted Green Synthesis of Binary/Ternary ZnxCo1−xFe2O4 (x = 0, 0.5, 1) Nanoparticles. Chem. Proc. 2024, 16, 29. https://doi.org/10.3390/ecsoc-28-20248
Chamani S, Khatamian M. Microwave-Assisted Green Synthesis of Binary/Ternary ZnxCo1−xFe2O4 (x = 0, 0.5, 1) Nanoparticles. Chemistry Proceedings. 2024; 16(1):29. https://doi.org/10.3390/ecsoc-28-20248
Chicago/Turabian StyleChamani, Sanaz, and Masoumeh Khatamian. 2024. "Microwave-Assisted Green Synthesis of Binary/Ternary ZnxCo1−xFe2O4 (x = 0, 0.5, 1) Nanoparticles" Chemistry Proceedings 16, no. 1: 29. https://doi.org/10.3390/ecsoc-28-20248
APA StyleChamani, S., & Khatamian, M. (2024). Microwave-Assisted Green Synthesis of Binary/Ternary ZnxCo1−xFe2O4 (x = 0, 0.5, 1) Nanoparticles. Chemistry Proceedings, 16(1), 29. https://doi.org/10.3390/ecsoc-28-20248





