1. Introduction
Currently, the photometric method is widely used in clinical laboratory practice to determine the level of urea in blood. This method is based on the formation of a colored complex between urea and diacetyl monoxime [
1]. More accessible for both home use and medical laboratories are analyzers that use the principle of amperometric enzyme biosensors. For example, the “3 in 1 Benecheck” glucometer (“BeneCheck”, New Taipei City, Taiwan) has test strips that can determine the uric acid, cholesterol, and glucose levels in the blood. Each test strip contains an electrode and an oxidoreductase enzyme. When a blood sample is added, a series of oxidation-reduction reactions occur, leading to a change in current strength. The microprocessor processes this information and displays the concentration of the biomarker on the screen. Since the determination of urea requires the use of an enzyme called urease, which is a hydrolase, in order to create a test strip, it is necessary to make modifications to ensure the fastest possible detection of ammonia formed during enzymatic hydrolysis. Therefore, the quantitative determination of this biomarker remains a task that has not yet been implemented on an industrial scale.
The use of the urease enzyme in amperometric biosensors has been described in previous studies [
2,
3]. The biosensor design [
2] utilizes a platinum electrode with a working surface coated with a copolymer membrane made from acrylonitrile, methyl methacrylate, and sodium vinylsulfonate. A layer of electroplated rhodium and immobilized urease in bovine serum albumin (BSA) are also applied to the electrode. The measured urea concentration range is between 0.1 and 2.67 mM. The measurement time per sample is approximately 15 s. Another amperometric biosensor design [
3] employs a graphite disk electrode coated with polyaniline and multi-walled carbon nanotubes. The enzyme urease is also immobilized on the electrode surface. This biosensor can detect urea concentrations ranging from 0.07 to 10 mM. The measurement duration for each sample is around 50 s. A significant disadvantage of these devices is their sensitivity, which allows for the monitoring of urea levels in the blood. The concentration of this biomarker in the blood at rest is typically between 2.5 and 9.2 mM, while the concentration in urine is significantly higher, typically ranging from 110 to 390 mM [
4]. The aim of this research is to expand the range of measurable concentrations by immobilizing the urease enzyme in a polymer that has been modified with safranin-BSA, in combination with fullerenes.
2. Materials and Methods
2.1. Reagents and Materials
Graphite powder (Fluka, Berlin, Germany) and a dialysis membrane (Roth, Dautphetal, Germany) with a cutoff of 14 kDa were used in combination with mineral oil (Helikon, Moscow, Russia) to create a working graphite electrode. Fullerene C60 nanopowder (SIA “MST-Nano EU”, St. Petersburg, Russia) with purity 99.9% and specific resistivity 1014 Ohm/m was used to modify the working electrode. To prepare the redox-active polymer, bovine serum albumin (BSA), safranin, and glutaraldehyde were used (Diaem, Moscow, Russia). The salts (Diaem, Moscow, Russia) used for the buffer solution were chemically pure.
2.2. Modification of the Working Electrode
The working electrode was fabricated by packing a plastic tube with a surface area of 6.3 mm2. A paste was prepared by combining 100 mg of graphite powder with 40 µL of mineral oil. A 10 μL of fullerene suspension (25%) was applied to the surface of a working electrode. Finally, 10 μL of the suspension of the redox polymer was applied to the electrode. The redox polymer was prepared by the following protocol: 0.0035 g of BSA was dissolved in 50 μL of potassium–sodium phosphate buffer (pH 6.8), and 6 μL of a saturated aqueous safranin solution was added with thorough mixing. Following this, 7.5 μL of glutaraldehyde was introduced, and the mixture was shaken for 30 s. The working electrode was then covered with a dialysis membrane and fixed with a plastic ring.
2.3. Biosensor Fabrication and Measurement
A CS310M potentiostat (Corrtest Instruments, Wuhan, China) was employed to capture the signal from the biosensor system, monitoring the relationship between current and time. The electrodes were linked to the electrochemical detector, and the measurements were conducted at a potential of +275 mV. A silver chloride electrode was used as a reference electrode during the process.
Measurements were conducted while stirring with a magnetic stirrer at a temperature of 20 °C and a total cell volume of 5 cm3, filled with a potassium–sodium phosphate buffer, maintaining a pH of 6.8. When a stable current was achieved, a urea solution was introduced into the cell, and the catalytic activity of the enzyme was determined based on the current amplitude. After each measurement, the electrochemical cell was rinsed with buffer solution.
To estimate the urea concentration in the samples, a biosensor was calibrated using a standard solution of urea. After taking into account the dilution factor, the urea content of the test samples was calculated using a calibration curve that was pre-constructed on the day of the analysis.
3. Results and Discussion
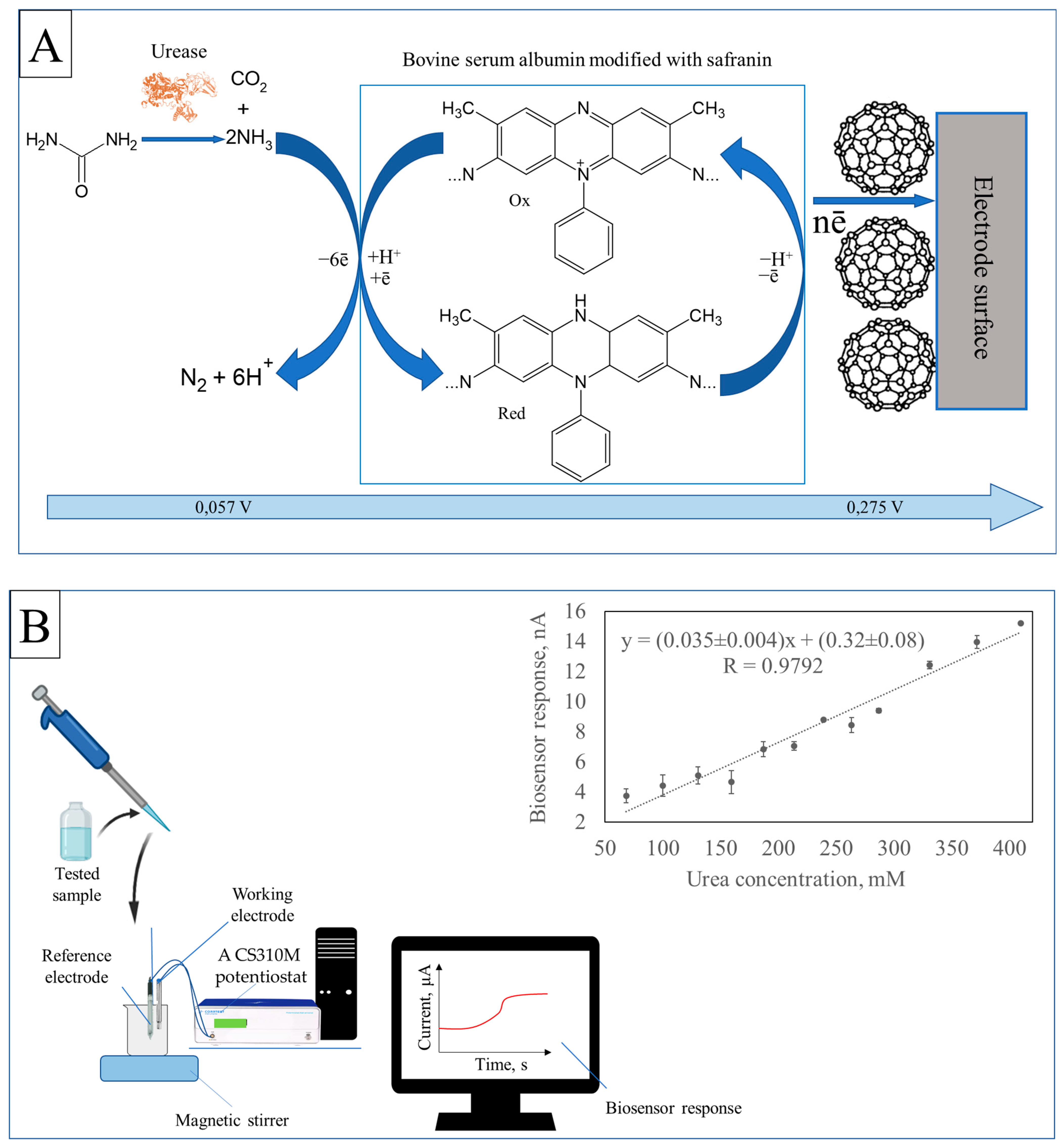
The principle of generating an analytical signal and the calibration dependence of the biosensor response on the concentration of urea in the solution are shown in
Figure 1.
According to
Figure 1A, when a sample containing urea is added, there is a reaction between the component being analyzed and immobilized urease in the near-electrode space of the working electrode. This enzyme catalyzes the hydrolysis of urea, producing ammonia and carbon dioxide. Ammonia interacts with safranin, which has been introduced into the BSA structure. At an applied potential, the polymer is oxidized, causing a change in current strength in the system. The difference in current strength before and after adding the sample is recorded as the biosensor’s analytical signal (
Figure 1A). The magnitude of this signal is proportional to the concentration of urea in the original sample (
Figure 1B). This biosensor can detect urea concentrations ranging from 68 to 410 mM (
Figure 1B), allowing for accurate measurement.
The increase in the upper limit of detectable concentrations, compared to analogues [
2,
3], is associated with a change in the Michaelis constant of the urease enzyme due to its immobilization. The components of the working electrode used in the biosensor change the microstructure of the enzyme, reducing its affinity for urea and requiring a higher concentration of substrate to achieve the same catalytic activity [
5]. Fullerene enhances the volume of the bioelectrocatalytic layer, while the fixation of safranin on BSA enhances the interaction between the enzyme and the electrode surface. These changes to the enzyme also alter the Michaelis constant, leading to an increase in the detectable concentration range. The obtained range allows for the further use of this biosensor in non-invasive monitoring of urea levels in urine.
4. Conclusions
This project successfully developed a biosensor for the rapid monitoring of urea levels, which is essential for the early detection of chronic conditions. The biosensor utilizes urease immobilized on a composite of bovine serum albumin (BSA), safranin (SAF), and fullerene, integrated into a modified graphite paste electrode. With the incorporation of a silver chloride reference electrode and a potentiostat set at +0.275 V, the sensor demonstrates its capability to accurately measure urea concentrations in urine within the range of 68 to 410 mM. This advancement in biosensor technology holds significant promise for clinical applications in health monitoring.
Despite the promising results of the developed biosensor for urea monitoring, there are several limitations to consider. Firstly, the sensor’s performance may be affected by potential interferences from other substances present in urine, which could lead to inaccurate readings. Additionally, the stability and longevity of the immobilized urease on the bionanocomposite may vary over time, potentially impacting the sensor’s reliability in long-term applications. Furthermore, the biosensor requires a controlled pH of 6.8 for optimal performance, which may not always be achievable in real-world samples. Lastly, while the sensor effectively measures urea concentrations from 68 to 410 mM, it may not be suitable for detecting lower or higher concentrations outside this range, limiting its applicability in certain clinical scenarios.
Author Contributions
Conceptualization, A.K.; data curation, A.K.; formal analysis, T.L. and M.G.; funding acquisition, A.K.; investigation, A.K. and T.L.; methodology, A.K. and M.G.; project administration, A.K. and M.G.; resources, A.K. and T.L.; supervision, A.K.; visualization, A.K., T.L. and M.G.; writing—original draft, A.K.; writing—review and editing, T.L. and M.G. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding authors.
Acknowledgments
The authors would like to express their gratitude to the Research Center “BioChemTech” for providing all the necessary equipment and facilities that made this research possible. This support and these resources were invaluable in carrying out our experiments and achieving our project goals.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Botewad, S.N.; Gaikwad, D.K.; Girhe, N.B.; Thorat, H.N.; Pawar, P.P. Urea biosensors: A comprehensive review. Biotechnol. Appl. Biochem. 2023, 70, 485–501. [Google Scholar] [CrossRef] [PubMed]
- Velichkova, Y.; Ivanov, Y.; Marinov, I.; Ramesh, R.; Kamini, N.; Dimcheva, N.; Horozova, E.; Godjevargova, T. Amperometric electrode for determination of urea using electrodeposited rhodium and immobilized urease. J. Mol. Catal. B Enzym. 2011, 69, 168–175. [Google Scholar] [CrossRef]
- Meibodi AS, E.; Haghjoo, S. Amperometric urea biosensor based on covalently immobilized urease on an electrochemically polymerized film of polyaniline containing MWCNTs. Synth. Met. 2014, 194, 1–6. [Google Scholar] [CrossRef]
- Jacobs, C.B.; Peairs, M.J.; Venton, B.J. Carbon nanotube based electrochemical sensors for biomolecules. Anal. Chim. Acta 2011, 662, 105–127. [Google Scholar] [CrossRef] [PubMed]
- Gergeroglu, H.; Yildirim, S.; Ebeoglugil, M.F. Nano-carbons in biosensor applications: An overview of carbon nanotubes (CNTs) and fullerenes (C60). SN Appl. Sci. 2020, 2, 603. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).