Synthesis and In Vitro Antibacterial Studies of Two New Hydrazone Derivatives †
Abstract
1. Introduction
2. Methods
2.1. Synthesis and Characterization
2.1.1. Reagents and Equipment Used for Synthesis
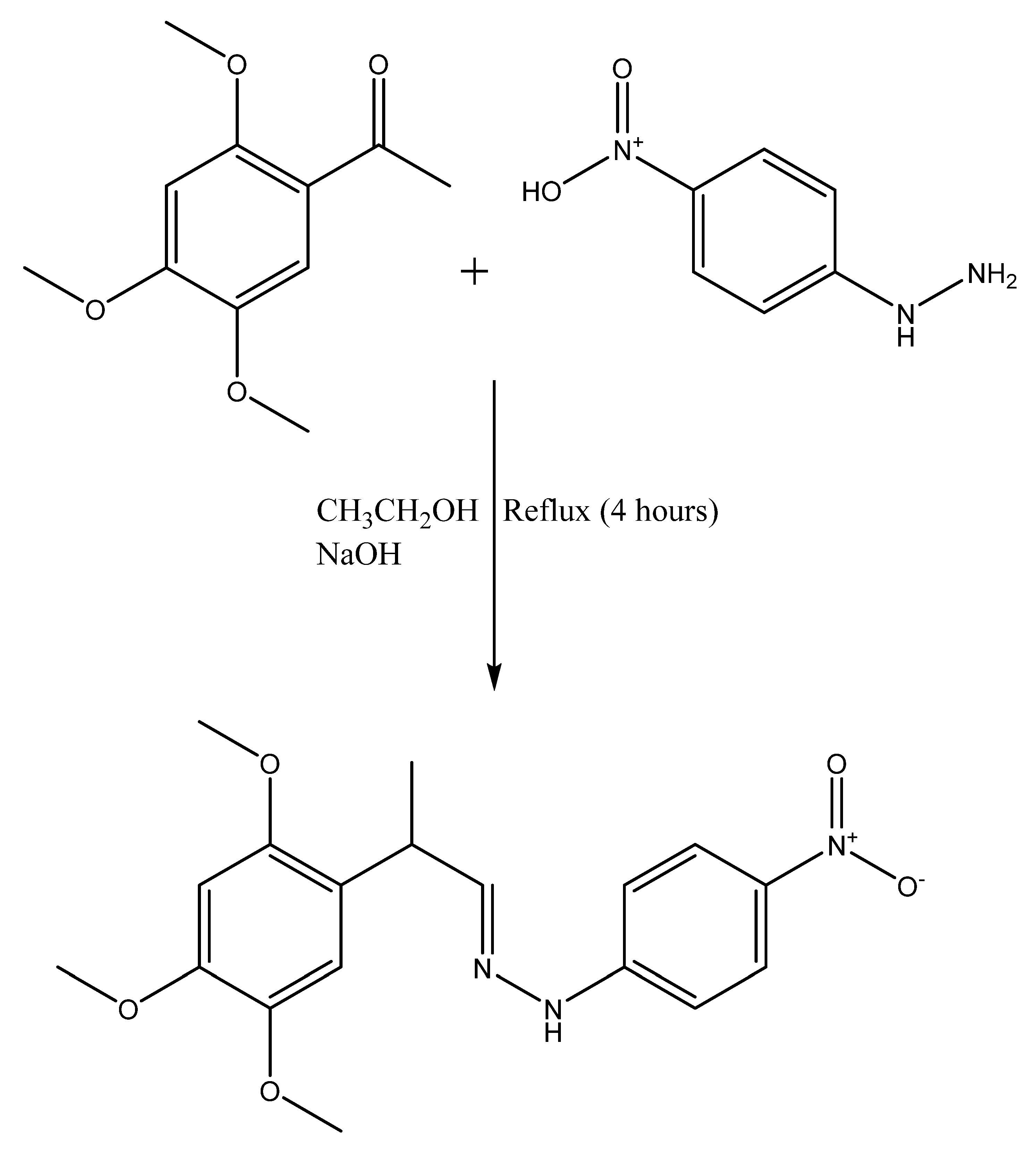
2.1.2. The General Procedure for the Synthesis of Hydrazone Derivatives (H3 and H4)
2.2. Evaluation of Antibacterial Activity
2.2.1. Procedure for Agar Diffusion Method
2.2.2. Procedure for Broth Dilution Method
3. Results and Discussions
3.1. Synthesis and Characterization
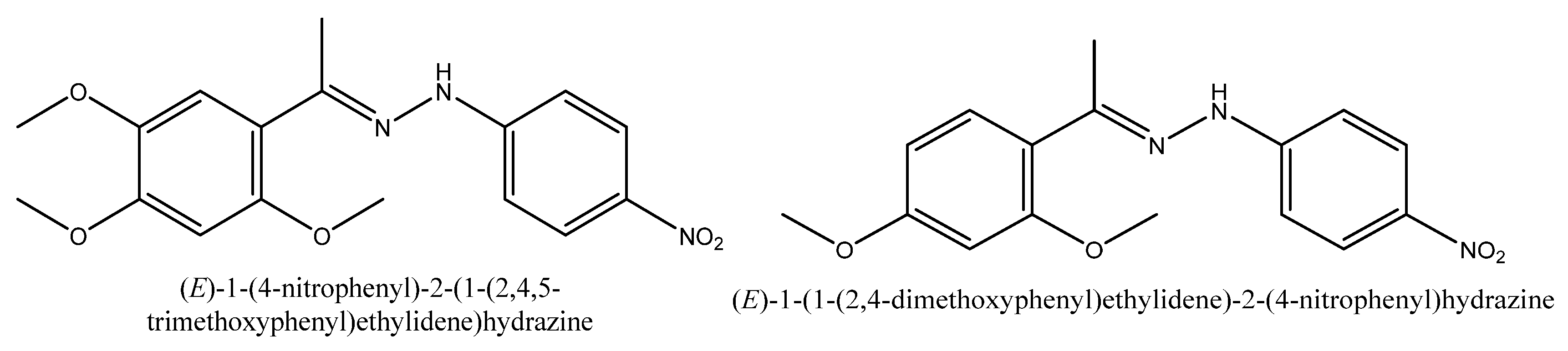
3.1.1. (E)-1-(4-nitrophenyl)-2-(1-(2,4,5-trimethoxyphenyl)ethylidene) Hydrazine (H3)
3.1.2. (E)-1-(4-nitrophenyl)-2-(1-(2,4,-dimethoxyphenyl)ethylidene) Hydrazine (H3)
3.2. Antibacterial Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Debta, P.; Swain, S.K.; Soyab, T.; Sahu, M.C.; Lenka, S. Microbial Infectious Disease: A Mini Review Microbial Infectious Disease: A Mini Review. Indian J. Forensic Med. Toxicol. 2020, 14, 8389–8393. [Google Scholar]
- Sant, A.P.; Nascimento, L.C.; Martins, C.S.; Araújo, J.M.; De Maria, T.; Correia, S.; Cavalcanti, S. Antimicrobial Activity and Phytochemical Analysis of Organic Extracts from Cleome spinosa Jaqc. Front. Microbiol. 2016, 7, 1–10. [Google Scholar] [CrossRef]
- Xu, P.; Li, W.; Xie, J.; Zhu, C. Exploration of C-H Transformations of Aldehyde Hydrazones: Radical Strategies and beyond. Acc. Chem. Res. 2018, 51, 484–495. [Google Scholar] [CrossRef] [PubMed]
- Evranos Aksöz, B.; Gürpinar, S.S.; Eryilmaz, M. Antimicrobial activities of some pyrazoline and hydrazone derivatives. Turk. J. Pharm. Sci. 2020, 17, 500–505. [Google Scholar] [CrossRef]
- Nasr, T.; Bondock, S.; Youns, M. Anticancer activity of new coumarin substituted hydrazide-hydrazone derivatives. Eur. J. Med. Chem. 2014, 76, 539–548. [Google Scholar] [CrossRef]
- Sarkar, S.; Siddiqui, A.A.; Saha, S.J.; De, R.; Mazumder, S.; Banerjee, C.; Iqbal, M.S.; Nag, S.; Adhikari, S.; Bandyopadhyay, U. Antimalarial activity of small-molecule benzothiazole hydrazones. Antimicrob. Agents Chemother. 2016, 60, 4217–4228. [Google Scholar] [CrossRef]
- Thomas, A.B.; Nanda, R.K.; Kothapalli, L.P.; Hamane, S.C. Synthesis and biological evaluation of Schiff’s bases and 2-azetidinones of isonocotinyl hydrazone as potential antidepressant and nootropic agents. Arab. J. Chem. 2016, 9, S79–S90. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef]
- Senkardes, S.; Kaushik-Basu, N.; Durmaz, I.; Manvar, D.; Basu, A.; Atalay, R.; Guniz Kucukguzel, S. Synthesis of novel diflunisal hydrazide-hydrazones as anti-hepatitis C virus agents and hepatocellular carcinoma inhibitors. Eur. J. Med. Chem. 2016, 108, 301–308. [Google Scholar] [CrossRef]
- Bisht, N.; Singh, B.K. Structure based virtual screening, molecular docking studies and modification of hydantoin nucleus analogues as anticonvulsants. Indian J. Chem.-Sect. B Org. Med. Chem. 2018, 57, 1514–1525. [Google Scholar]
- Kabir, E.R.; Mustafa, N.; Nausheen, N.; Sharif Siam, M.K.; Syed, E.U. Exploring existing drugs: Proposing potential compounds in the treatment of COVID-19. Heliyon 2021, 7, e06284. [Google Scholar] [CrossRef] [PubMed]
- El-Tombary, A.; El-Hawash, A.M. Synthesis, Antioxidant, Anticancer and Antiviral Activities of Novel Quinoxaline Hydrazone Derivatives and their Acyclic C-Nucleosides. Med. Chem. 2014, 10, 521–532. [Google Scholar] [CrossRef] [PubMed]
- Masson, E.; All, S.A.S.; Infectious, I. Antimicrobial activity and a SAR study of some novel benzimidazole derivatives bearing hydrazone moiety. Eur. J. Med. Chem. 2010, 45, 3293–3298. [Google Scholar] [CrossRef]
- Popiołek, Ł.; Biernasiuk, A. New Hydrazides and Hydrazide-Hydrazones of 2,3-Dihalogen Substituted Propionic Acids: Synthesis and in vitro Antimicrobial Activity Evaluation. Chem. Biodivers. 2017, 14, e1700075. [Google Scholar] [CrossRef]
- Binesh Kumar, J.; Jai Devi Dubey, A.; Tufail, A.; Taxak, B. Investigation of antituberculosis, antimicrobial, anti-inflammatory efficacies of newly synthesized transition metal (II) complexes of hydrazone ligands: Structural elucidation and theoretical studies. Sci. Rep. 2023, 13, 15906. [Google Scholar] [CrossRef]
- MacHakanur, S.S.; Patil, B.R.; Badiger, D.S.; Bakale, R.P.; Gudasi, K.B.; Annie Bligh, S.W. Synthesis, characterization and anticancer evaluation of novel tri-arm star shaped 1,3,5-triazine hydrazones. J. Mol. Struct. 2012, 1011, 121–127. [Google Scholar] [CrossRef]
- Abdelrahman, M.A.; Salama, I.; Gomaa, M.S.; Elaasser, M.M.; Abdel-Aziz, M.M.; Soliman, D.H. Design, synthesis and 2D QSAR study of novel pyridine and quinolone hydrazone derivatives as potential antimicrobial and antitubercular agents. Eur. J. Med. Chem. 2017, 138, 698–714. [Google Scholar] [CrossRef]
- Popiołek, Ł.; Biernasiuk, A.; Berecka, A.; Gumieniczek, A.; Malm, A.; Wujec, M. New hydrazide–hydrazones of isonicotinic acid: Synthesis, lipophilicity and in vitro antimicrobial screening. Chem. Biol. Drug Des. 2018, 91, 915–923. [Google Scholar] [CrossRef]
- Popiołek, Ł. Updated Information on Antimicrobial Activity of Hydrazide—Hydrazones. Int. J. Mol. Sci. 2021, 22, 9389. [Google Scholar] [CrossRef]
- Kumar, N.; Chauhan, L.S.; Bera, R. Synthesis, analgesic and anti-inflammatory activities of chalconyl-incorporated hydrazone derivatives of mefenamic acid. Med. Chem. Res. 2015, 24, 2580–2590. [Google Scholar] [CrossRef]
- Sharma, P.C.; Sharma, D.; Sharma, A.; Saini, N.; Goyal, R.; Ola, M.; Chawla, R.; Thakur, V.K. Hydrazone comprising compounds as promising anti-infective agents: Chemistry and structure-property relationship. Mater. Today Chem. 2020, 18, 100349. [Google Scholar] [CrossRef]
- Inam, A.; Siddiqui, S.M.; Macedo, T.S.; Moreira, D.R.M.; Leite, A.C.L.; Soares, M.B.P.; Azam, A. Design, synthesis and biological evaluation of 3-[4-(7-chloro-quinolin-4- yl)-piperazin-1-yl]-propionic acid hydrazones as antiprotozoal agents. Eur. J. Med. Chem. 2014, 75, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, I.; Hilpert, K.; Hancock, R.E.W. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Rasras, A.J.M.; Al-Tel, T.H.; Al-Aboudi, A.F.; Al-Qawasmeh, R.A. Synthesis and antimicrobial activity of cholic acid hydrazone analogues. Eur. J. Med. Chem. 2010, 45, 2307–2313. [Google Scholar] [CrossRef]
- Talebi Bezmin Abadi, A.; Rizvanov, A.A.; Haertlé, T.; Blatt, N.L. World Health Organization Report: Current Crisis of Antibiotic Resistance. BioNanoScience 2019, 9, 778–788. [Google Scholar] [CrossRef]
- Kamboj, S. Scholars Research Library Therapeutic Review Exploring Antimicrobial Potential of. Der Pharma Chem. 2014, 3, 250–268. [Google Scholar]
| Organism | Compound | Concentrations of Compounds (mg/mL) | Cipro (mg/mL) | |||
|---|---|---|---|---|---|---|
| 50 | 25 | 12.5 | 6.25 | 0.05 | ||
| S. aureus | H3 | 19 | 15 | 12 | 11 | 20 |
| H4 | 17 | 14 | 11 | 0 | 20 | |
| E. coli | H3 | 18 | 13 | 11 | 0 | 24 |
| H4 | 16 | 14 | 12 | 0 | 24 | |
| Organism | Compound | Concentrations (mg/mL) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 50 | 25 | 12.5 | 6.25 | 3.125 | 1.56 | 0.78 | 0.39 | 0.2 | 0.1 | ||
| S. aureus | H3 | − | + | + | + | + | + | + | + | + | + |
| H4 | + | + | + | + | + | + | + | + | + | + | |
| E. coli | H3 | − | + | + | + | + | + | + | + | + | + |
| H4 | + | + | + | + | + | + | + | + | + | + | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karimatu, H.; Yunusa, I.A.; Musa, M.A.; Nasiru, H.A.; Auwal, H.S.; Maryam, A. Synthesis and In Vitro Antibacterial Studies of Two New Hydrazone Derivatives. Chem. Proc. 2024, 16, 118. https://doi.org/10.3390/ecsoc-28-20138
Karimatu H, Yunusa IA, Musa MA, Nasiru HA, Auwal HS, Maryam A. Synthesis and In Vitro Antibacterial Studies of Two New Hydrazone Derivatives. Chemistry Proceedings. 2024; 16(1):118. https://doi.org/10.3390/ecsoc-28-20138
Chicago/Turabian StyleKarimatu, Hamza, Idris Abdullahi Yunusa, Muhammad Aliyu Musa, Hamza Asmau Nasiru, Hamza Sa’adatu Auwal, and Abdullahi Maryam. 2024. "Synthesis and In Vitro Antibacterial Studies of Two New Hydrazone Derivatives" Chemistry Proceedings 16, no. 1: 118. https://doi.org/10.3390/ecsoc-28-20138
APA StyleKarimatu, H., Yunusa, I. A., Musa, M. A., Nasiru, H. A., Auwal, H. S., & Maryam, A. (2024). Synthesis and In Vitro Antibacterial Studies of Two New Hydrazone Derivatives. Chemistry Proceedings, 16(1), 118. https://doi.org/10.3390/ecsoc-28-20138





