Design, Synthesis and Characterization of a Phosphino-Azine Ligand †
Abstract
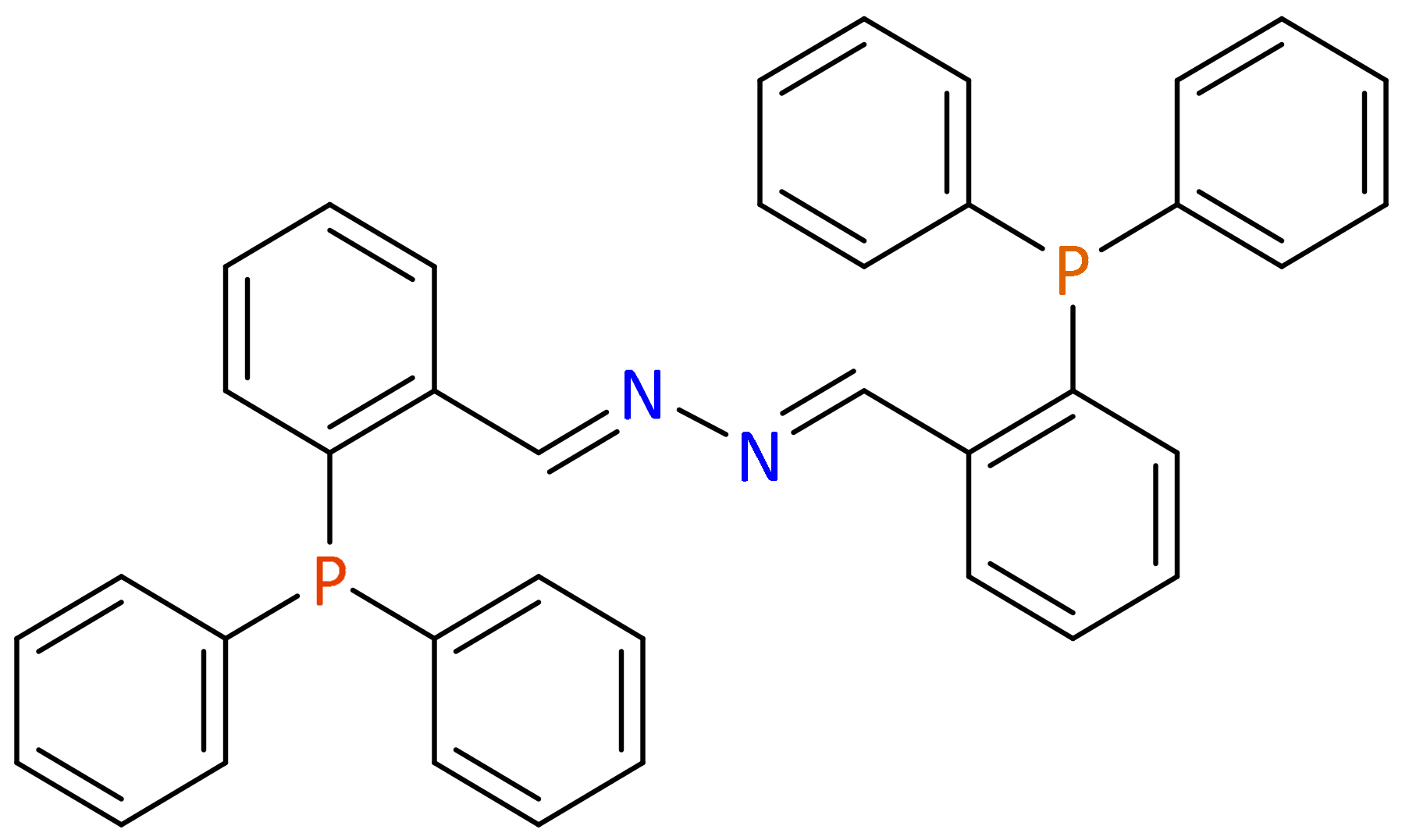
1. Introduction
2. Experimental Section
2.1. Reactants and Solvents
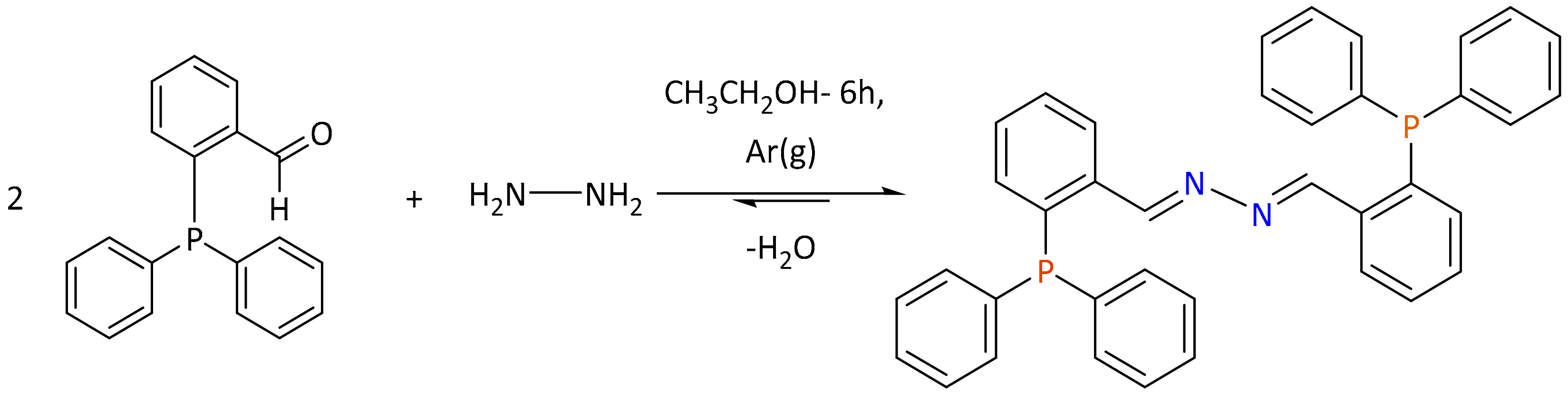
2.2. Synthesis and Characterization of the Bisthiosemicarbazone Ligand LP
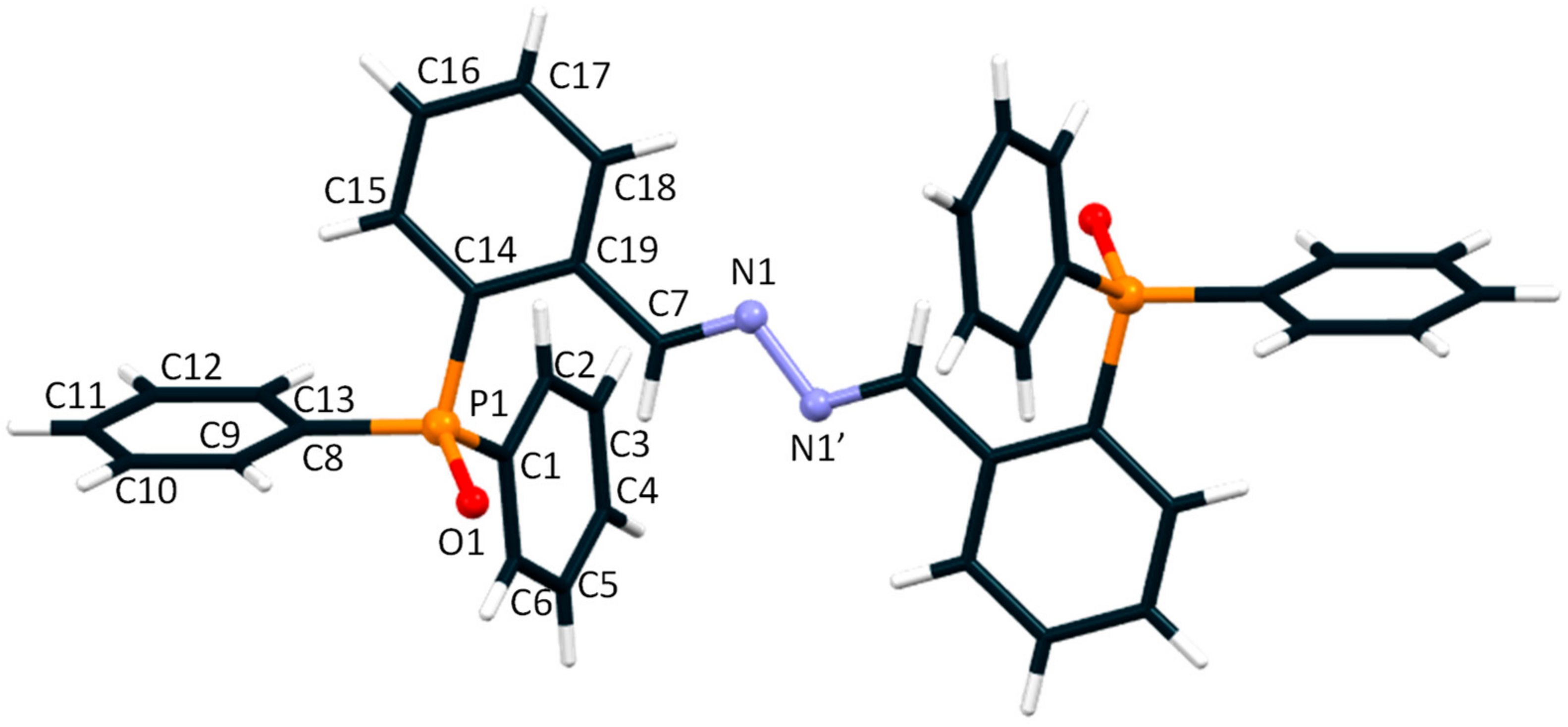
2.3. Crystallographic Data of LPO2·2CH3OH
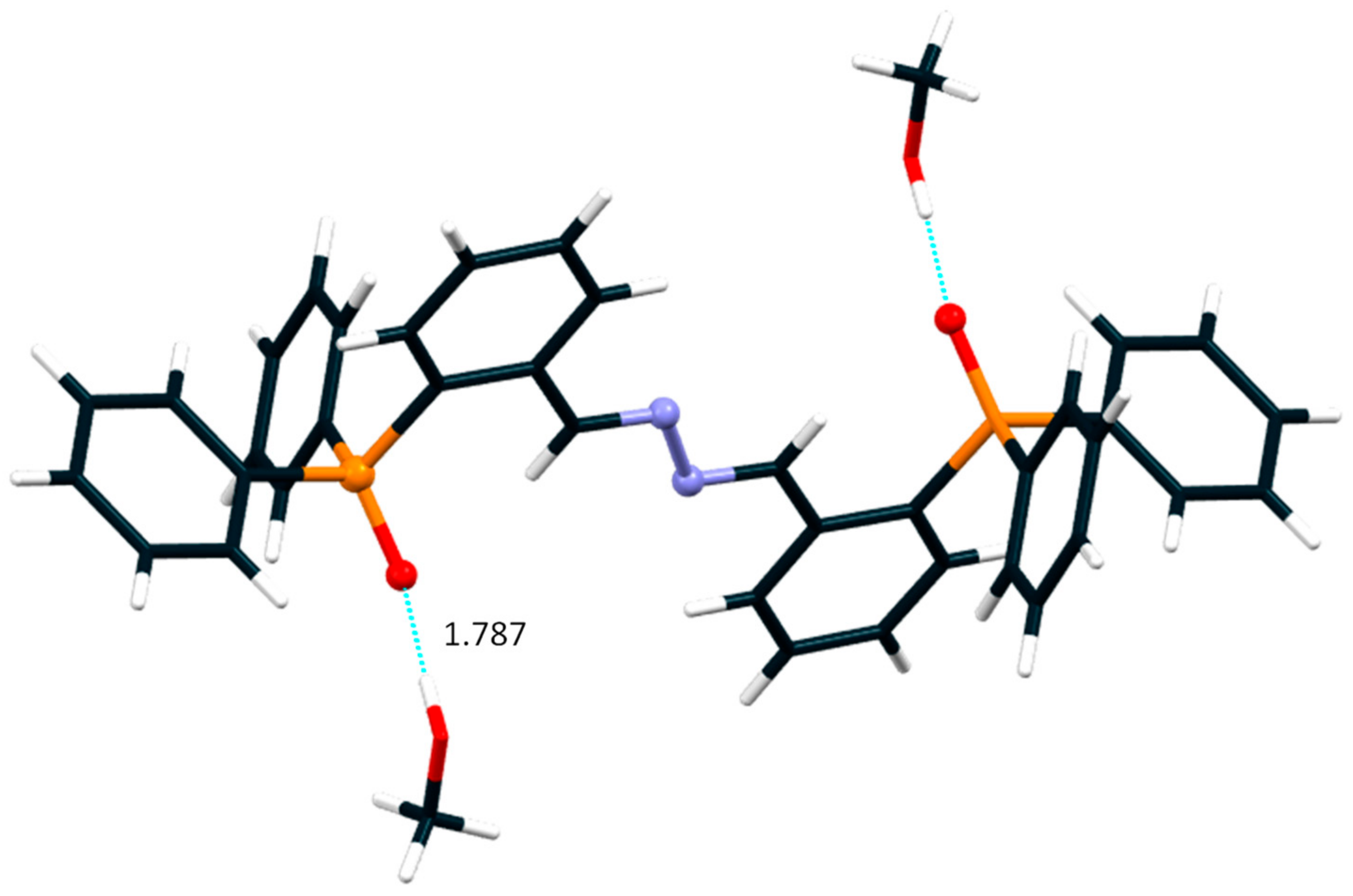
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nalwa, H.S.; Kakuta, A.; Mukoh, A. Third-order nonlinear optical properties of processable polyazine thin films. J. Appl. Phys. 1993, 73, 4743–4745. [Google Scholar] [CrossRef]
- Euler, W.B.; Cheng, M.; Zhao, C. End-group effects on the structure and spectroscopy of oligoazines. Chem. Mater. 1999, 11, 3702–3708. [Google Scholar] [CrossRef]
- Haggerty, W.J., Jr.; Cheng, C.C. Antitumor activity of some azine and hydrazone derivatives of 1,4-dimethoxy-2-butanone. J. Med. Chem. 1970, 13, 574–575. [Google Scholar] [CrossRef] [PubMed]
- Khodair, A.I.; Bertrand, P. A new approach to the synthesis of substituted 4 imidazolidinones as potential antiviral and antitumor agents. Tetrahedron 1998, 54, 4859–4872. [Google Scholar] [CrossRef]
- Yousaf, M.; Pervaiz, M.; Sagir, M.; Uz-Zaman, A.; Mushtaq, M.; Naz, M.Y. Synthesis of tetradentate schiff base derivatives of transition bimetallic complexes as antimicrobial agents. J. Chin. Chem. Soc. 2013, 60, 1150–1155. [Google Scholar] [CrossRef]
- Nemytov, A.I.; Utepova, I.A.; Kiskin, M.A.; Efimov, N.N.; Fedin, M.V.; Eremenko, I.L.; Musikhina, A.A.; Slepukhin, P.A.; Chupakhin, O.N. Synthesis, structure and magnetic properties of binuclear 3d-metal complexes of new 3-(2-pyridyl)-6-phenyl-1,2,4-triazine derivative. Polyhedron 2021, 193, 114901. [Google Scholar] [CrossRef]
- Noshiranzadeh, N.; Bikas, R.; Emami, M.; Siczek, M.; Lis, T. Oxidative coupling of 2-naphthol catalyzed by a new methoxido bridged dinuclear oxidovanadium(V) complex. Polyhedron 2016, 111, 167–172. [Google Scholar] [CrossRef]
- Kuźniarska-Biernacka, I.; Raposo, M.M.M.; Batista, R.M.F.; Soares, O.S.G.P.; Pereira, M.F.R.; Parpot, P.; Oliveira, C.; Skiba, E.; Jartych, E.; Fonseca, A.M. Binuclear furanyl-azine metal complexes encapsulated in NaY zeolite as efficiently heterogeneous catalysts for phenol hydroxylation. J. Mol. Struct. 2020, 1206, 127687. [Google Scholar] [CrossRef]
- Fernández-Fariña, S.; González-Barcia, L.M.; Romero, M.J.; García-Tojal, J.; Marcelino, M.; Seco, J.M.; Zaragoza, G.; Martínez-Calvo, M.; González-Noya, A.M.; Pedrido, R. Conversion of double-tetranuclear cluster silver helicate into a dihelicate via a rare desulfurization process. Org. Chem. Front. 2022, 9, 531. [Google Scholar] [CrossRef]
- González-Barcia, L.M.; Fernández-Fariña, S.; Rodríguez-Silva, L.; Bermejo, M.R.; González-Noya, A.M.; Pedrido, R. Comparative study of the antitumoral activity of phosphine-thisemicarbazone gold(I) complexes obtained by different methodologies. J. Inorg. Biochem. 2020, 203, 110931. [Google Scholar] [CrossRef] [PubMed]
| Main Bond Distances (Å) | |||
| P1-O1 | 1.4946 (14) | P1-C8 | 1.806 (2) |
| P1-C14 | 1.795 (2) | N1-N1′ | 1.413 (3) |
| Angles (o) | |||
| C7-N1-N1′ | 111.10 (2) | O1-P1-C14 | 112.62 (9) |
| O1-P1-C8 | 110.97 (8) | O1-P1-C14 | 113.31 (8) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barreiro-Sisto, U.; Maneiro, M.; Pedrido, R. Design, Synthesis and Characterization of a Phosphino-Azine Ligand. Chem. Proc. 2024, 16, 105. https://doi.org/10.3390/ecsoc-28-20129
Barreiro-Sisto U, Maneiro M, Pedrido R. Design, Synthesis and Characterization of a Phosphino-Azine Ligand. Chemistry Proceedings. 2024; 16(1):105. https://doi.org/10.3390/ecsoc-28-20129
Chicago/Turabian StyleBarreiro-Sisto, Uxía, Marcelino Maneiro, and Rosa Pedrido. 2024. "Design, Synthesis and Characterization of a Phosphino-Azine Ligand" Chemistry Proceedings 16, no. 1: 105. https://doi.org/10.3390/ecsoc-28-20129
APA StyleBarreiro-Sisto, U., Maneiro, M., & Pedrido, R. (2024). Design, Synthesis and Characterization of a Phosphino-Azine Ligand. Chemistry Proceedings, 16(1), 105. https://doi.org/10.3390/ecsoc-28-20129







