Abstract
Researchers have linked Chlamydia pneumoniae (C. pneumoniae), a type of bacteria that cannot survive outside of cells and is resistant to Gram staining, to many autoimmune diseases. People hypothesized that C. pneumoniae had a harmful function due to its tendency to inhabit human endothelium and epithelial tissue. This study implemented multiple bioinformatics tools and databases to understand the possible function of the CT670 hypothetical protein of C. pneumoniae. The physicochemical parameters showed the protein’s half-life in different media. These parameters also displayed the protein’s theoretical isoelectric point, aliphatic index, GRAVY value, extinction coefficient, instability index, and the amino acids and atoms that it comprises. Amino acid composition measured the percentage of amino acids present in the selected protein, with glutamate demonstrated as the greatest proportion. Moreover, hydrogen was the most abundant ratio in terms of the atomic composition of the protein, followed by carbon, oxygen, nitrogen, and sulfur. The PPI networks reveal its potential primary and secondary interactions with other proteins. We modeled and assessed the secondary and tertiary structures to understand the nature of the selected protein. Computational functional analysis predicted that the protein would be a chaperone effector. By designing and developing drugs and vaccines, we can use this protein as a target for further analysis to combat diseases caused by C. pneumoniae.
1. Introduction
C. pneumoniae, a kind of bacteria that cannot survive outside of cells and has a negative reaction to the Gram stain, is a common respiratory infection. It is often responsible for respiratory disorders in humans, such as pneumonia [1]. Despite the complete sequencing of the C. pneumoniae genome, there is still a lack of knowledge about the processes of acute infection, target cell activation, and the discovery of possible chlamydial virulence factors. Interestingly, several groups of patients with atherosclerosis have shown that the existing antibiotic treatments for acute chlamydial infection are ineffective in achieving positive clinical outcomes [2].
However, in addition to respiratory infections, C. pneumoniae also plays a role in the development of various inflammatory conditions, including asthma, COPD, lung cancer, neurological disorders like multiple sclerosis, Alzheimer’s disease, and schizophrenia, as well as arthritis and atherosclerosis. Therefore, the healthcare professional must possess the ability to immediately identify, assess, and manage this disease in order to prevent any associated problems [3,4,5].
2. Methods
2.1. Sequence Retrieval
The amino acid sequence of the CT670 protein of C. pneumoniae was collected from the NCBI protein database with the accession number BAA88656 (version number: BAA88656.1) [6].
2.2. Physicochemical Properties
The ProtParam web-based tool was used for the determination of the physicochemical properties of the CT670 protein with default parameters [7].
2.3. Protein–Protein Interaction (PPI) and Functional Analysis
The PPI was determined by utilizing the STRING database (v.12) [8] and visualized by Cytoscape software (v.3.10.2) [9]. Functional analysis was performed using the CD-Search tool of the NCBI [10].
2.4. Secondary and Tertiary Structural Assessment
SOPMA web-based software was used with default options (width for output: 70; similarity threshold: 8; and window width: 17) to determine the secondary structural parameters [11]. The 3D structure was predicted using the Swiss-Model server [12]. In addition, the Swiss-Model’s assessment tool and the ProSA-Web were implemented to verify the modeled structure of the protein [12,13,14].
3. Results and Discussion
3.1. Protein Sequence Retrieval and Physicochemical Properties Determination
Protein sequence analysis is a fundamental and essential approach for evaluating biological information. Given the significance of proteins, protein sequence benchmarks play a particularly vital role [15,16]. The obtained sequence is consisted of 168 amino acids retrieved in FASTA format from the NCBI database (GenBank accession: BAA88656.1). It located to the locus BAA88656 of C. pneumoniae [6].
The chemical composition of the constituent amino acids determines the physicochemical characteristics of proteins [15]. The ProtParam anticipated the protein’s molecular weight of 19,882.89 Dalton and the isoelectric point (pI) of 8.84. There was an increased amount of protein solubility and electrophoretic separation, as measured by the isoelectric point (pI), which is greater than 7.0 [17,18]. Moreover, the amino acid composition (Figure 1a) and the atomic composition (Figure 1b) were also measured. The negatively charged residues including Asp and Glu were 38, while the positively charged ones (Arg and Lys) were 41. One way to quantify the degree to which a protein absorbs light at a given wavelength is by looking at its extinction coefficient, which is another name for its molar absorption or absorption coefficient. For every given protein molecule, there is an individual extinction coefficient [19]. The extinction coefficient of the selected protein was anticipated as 9970 M−1 cm−1. Additionally, the estimated half-lives were 30 h (mammalian reticulocytes, in vitro), >20 h (yeast, in vivo), and >10 h (Escherichia coli, in vivo). In a laboratory setting, one can evaluate proteins for their potential stability using the instability index [20]. The predicted instability index of the targeted protein was 62.24. The aliphatic index refers to the percentage of a protein’s total volume that its aliphatic side chains account for [21,22]. Computational methods measured the aliphatic index at 83.57. The GRAVY value is one way to quantify the overall hydrophobicity of a protein or peptide [23,24]. The GRAVY value was measured at −1.151 of the selected hypothetical protein.
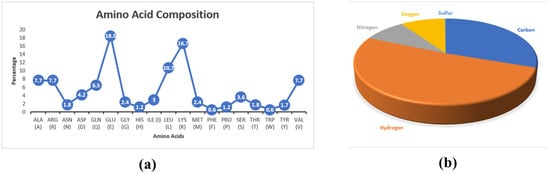
Figure 1.
(a) Amino acid composition of the selected protein. (b) Atomic composition of the protein. Hydrogen (n = 1460) is the most abundant chemical element, followed by carbon (n = 865), oxygen (n = 271), nitrogen (n = 254), and sulfur (n = 4).
3.2. Protein–Protein Interaction (PPI) and Functional Analysis
Electrostatic forces, hydrogen bonds, and hydrophobic influence are some of the interactions that make PPIs possible. PPIs are specific physical contacts between two or more protein molecules, while interactions influence metabolic processes [25,26]. While performing the PPI network (Figure 2), the STRING program demonstrated 21 nodes, 128 edges, an average node degree of 12.2, a median clustering coefficient value of 0.777, and an enrichment p-value of less than 1.0 × 10−16. Moreover, the CD-Search tool predicted that the protein was a YscO-like protein. Some proteins, including Chlamydia trachomatis’s CT670, have unknown cellular roles despite having genes in a type III secretion gene cluster. The structures and/or functionalities of these proteins are still unknown; however, CT670 has many similarities with the Yersinia pestis YscO protein, such as the adjacent genes, size, charge, and secondary structure. CT670 interacts with CT671, which is a potential YscP homolog. It is possible that CT670 and CT671 function together as a chaperone–effector pair [27].
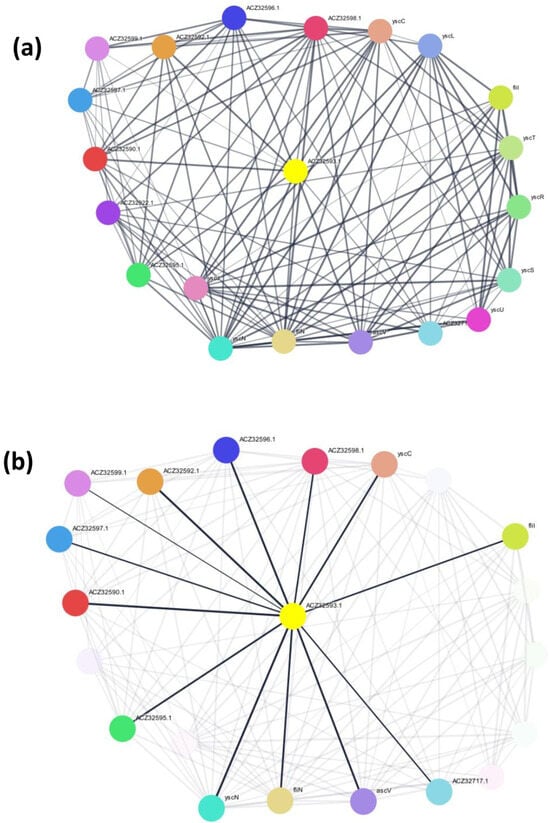
Figure 2.
(a) The PPI network of the selected hypothetical protein. The program selected ACZ32593.1 as an input to generate the network. It interacted with multiple proteins in both primary and secondary interactions, for example, with ACZ32592.1, yscN, ACZ32590.1, ACZ32596.1, ascV, ACZ32595.1, fliN, yscC, fliI, and ACZ32598.1. (b) The first-neighbor interactions of the selected protein.
3.3. Secondary and Tertiary Structural Assessment
The sequence of amino acids is the main building block. The term ‘secondary structure’ refers to the local interactions between parts of a polypeptide chain. It includes both α-helix and β-pleated sheet structures (Figure 3). Interactions between R groups are the primary drivers of tertiary structure, which is the overall three-dimensional folding [28,29]. The 3D structure was predicted based on the most suitable template following GMQE (0.95) and identity (84.43%) compared to the selected hypothetical protein. Moreover, the predicted structure was assessed by Z-score and Ramachandran plots (Figure 3).
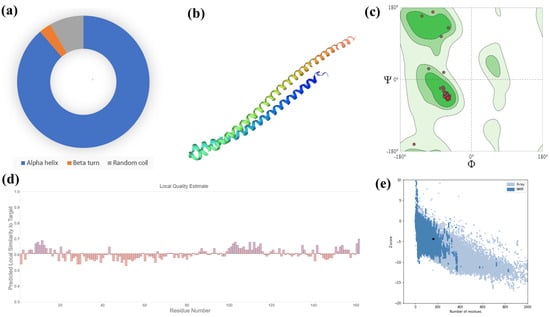
Figure 3.
(a) Secondary structural elements revealed that alpha helix (n = 149, 88.69%) was the most abundant, followed by random coil (n = 14, 8.33%) and beta turn (n = 5, 2.98%). (b) The 3D structure of the selected hypothetical protein. (c) Structural assessment by the Swiss-Model program. Ramachandran plots measured that 98.75% of amino acids were in the most favored regions and there were no Ramachandran outliers. (d) The ‘Local Quality Estimate’ values of the selected protein with other parameters including QMEAN (3.13), Cβ (5.75), solvation (4.40), and torsion (0.09). (e) Z-score obtained from the ProSA-web demonstrated as −4.3.
4. Conclusions
Researchers have linked C. pneumoniae, which cannot survive outside of cells and is resistant to Gram staining. Researchers postulated that C. pneumoniae’s propensity to reside in human endothelium and epithelial tissue played a detrimental role. This study utilized various bioinformatics methods and databases to elucidate the potential functionality of the CT670 putative protein of C. pneumoniae. The physicochemical properties indicated the protein’s half-life in various media. These metrics also indicated the protein’s theoretical isoelectric point, aliphatic index, GRAVY value, extinction coefficient, instability index, and the amino acids and atoms that make up the protein. The amino acid composition quantified the relative abundance of different amino acids in the chosen protein, with glutamate shown to be the most prevalent. Furthermore, hydrogen had the highest proportion in the atomic makeup of the protein, with carbon, oxygen, nitrogen, and sulfur following in descending order. The PPI networks demonstrated the possibility for both primary and secondary interactions with other proteins. We employed computational modeling techniques to analyze and evaluate the secondary and tertiary structures of the chosen protein in order to gain insights into its characteristics. Computational functional analysis anticipated that the protein would serve as part of a chaperone–effector pair. Through the process of designing and developing medications and vaccines, we can utilize this protein as a specific target for in-depth examination in order to treat diseases caused by C. pneumoniae.
Author Contributions
Conceptualization, A.S.M.S. and M.L.K.; methodology, A.S.M.S. and M.L.K.; software, A.S.M.S., T.A., U.S., K.N.U., M.M.H. and M.L.K.; validation, A.S.M.S., T.A., U.S., K.N.U., M.M.H. and M.L.K.; formal analysis, A.S.M.S., T.A. and U.S.; investigation, A.S.M.S., K.N.U., M.M.H. and M.L.K.; resources, A.S.M.S., K.N.U., M.M.H. and M.L.K.; data curation, A.S.M.S., T.A., U.S., K.N.U., M.M.H. and M.L.K.; writing—original draft preparation, A.S.M.S., T.A., U.S., K.N.U., M.M.H. and M.L.K.; writing—review and editing, A.S.M.S., K.N.U., M.M.H. and M.L.K.; visualization, A.S.M.S., T.A., U.S., K.N.U., M.M.H. and M.L.K.; supervision, M.L.K.; project administration, A.S.M.S. and M.L.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
COPD = Chronic obstructive pulmonary disease, GRAVY = Grand average of hydropathy, NCBI = National Center for Biotechnology Information, CD-Search = Conserved Domain Search, SOPMA = Self-Optimized Prediction Method with Alignment, FASTA = Fast Adaptive Shrinkage Threshold Algorithm, GMQE = Global Model Quality Estimation, QMEAN = Qualitative Model Energy Analysis, ProSA-web = Protein Structure Analysis.
References
- Premachandra, N.M.; Jayaweera, J. Chlamydia pneumoniae infections and development of lung cancer: Systematic review. Infect. Agent. Cancer 2022, 17, 11. [Google Scholar] [CrossRef]
- Krüll, M.; Maass, M.; Suttorp, N.; Rupp, J. Chlamydophila pneumoniae. Mechanisms of target cell infection and activation. Thromb. Haemost. 2005, 94, 319–326. [Google Scholar]
- Puri, B.K.; Lee, G.S.; Schwarzbach, A. The Role of Chlamydia pneumoniae in the Aetiology of Autoimmune Diseases. Cureus 2023, 15, e49095. [Google Scholar] [CrossRef] [PubMed]
- Hashemian, S.M.M.; Madani, S.A.; Allymehr, M.; Talebi, A. A molecular survey of Chlamydia spp. infection in commercial poultry and detection of Chlamydia pneumoniae in a commercial turkey flock in Iran. Vet. Med. Sci. 2023, 9, 2168–2175. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Lin, L.; Zhang, N.; Yang, Y. Adenovirus and Mycoplasma pneumoniae co-infection as a risk factor for severe community-acquired pneumonia in children. Front. Pediatr. 2024, 12, 1337786. [Google Scholar] [CrossRef]
- Sayers, E.W.; Beck, J.; Bolton, E.E.; Bourexis, D.; Brister, J.R.; Canese, K.; Comeau, D.C.; Funk, K.; Kim, S.; Klimke, W.; et al. Database resources of the National Center for Biotechnology Information. Nucleic Acids Res. 2021, 49, D10–D17. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, M.R.; Gasteiger, E.; Bairoch, A.; Sanchez, J.C.; Williams, K.L.; Appel, R.D.; Hochstrasser, D.F. Protein identification and analysis tools in the ExPASy server. Methods Mol. Biol. 1999, 112, 531–552. [Google Scholar]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The STRING database in 2023: Protein-protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 2023, 51, D638–D646. [Google Scholar] [CrossRef]
- Doncheva, N.T.; Morris, J.H.; Gorodkin, J.; Jensen, L.J. Cytoscape StringApp: Network Analysis and Visualization of Proteomics Data. J. Proteome Res. 2019, 18, 623–632. [Google Scholar] [CrossRef]
- Marchler-Bauer, A.; Anderson, J.B.; Cherukuri, P.F.; DeWeese-Scott, C.; Geer, L.Y.; Gwadz, M.; He, S.; Hurwitz, D.I.; Jackson, J.D.; Ke, Z.; et al. CDD: A Conserved Domain Database for protein classification. Nucleic Acids Res. 2005, 33, D192–D196. [Google Scholar] [CrossRef]
- Combet, C.; Blanchet, C.; Geourjon, C.; Deléage, G. NPS@: Network protein sequence analysis. Trends Biochem. Sci. 2000, 25, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; De Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef] [PubMed]
- Bienert, S.; Waterhouse, A.; de Beer, T.A.P.; Tauriello, G.; Studer, G.; Bordoli, L.; Schwede, T. The SWISS-MODEL Repository-new features and functionality. Nucleic Acids Res. 2017, 45, D313–D319. [Google Scholar] [CrossRef] [PubMed]
- Wiederstein, M.; Sippl, M.J. ProSA-web: Interactive web service for the recognition of errors in three-dimensional structures of proteins. Nucleic Acids Res. 2007, 35, W407–W410. [Google Scholar] [CrossRef]
- Akbari Rokn Abadi, S.; Abdosalehi, A.S.; Pouyamehr, F.; Koohi, S. An accurate alignment-free protein sequence comparator based on physicochemical properties of amino acids. Sci. Rep. 2022, 12, 11158. [Google Scholar] [CrossRef]
- Saikat, A.S.M.; Ripon, A. Structure Prediction, Characterization, and Functional Annotation of Uncharacterized Protein BCRIVMBC126_02492 of Bacillus cereus: An In Silico Approach. Am. J. Pure Appl. Biosci. 2020, 2, 104–111. [Google Scholar]
- Audain, E.; Ramos, Y.; Hermjakob, H.; Flower, D.R.; Perez-Riverol, Y. Accurate estimation of isoelectric point of protein and peptide based on amino acid sequences. Bioinformatics 2016, 32, 821–827. [Google Scholar] [CrossRef]
- Saikat, A.S.M. Computational approaches for molecular characterization and structure-based functional elucidation of a hypothetical protein from Mycobacterium tuberculosis. Genom. Inform. 2023, 21, e25. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.C.; von Hippel, P.H. Calculation of protein extinction coefficients from amino acid sequence data. Anal. Biochem. 1989, 182, 319–326. [Google Scholar] [CrossRef]
- Gamage, D.G.; Gunaratne, A.; Periyannan, G.R.; Russell, T.G. Applicability of Instability Index for In vitro Protein Stability Prediction. Protein Pept. Lett. 2019, 26, 339–347. [Google Scholar] [CrossRef]
- Ikai, A. Thermostability and aliphatic index of globular proteins. J. Biochem. 1980, 88, 1895–1898. [Google Scholar]
- Alam, M.M.; Saikat, A.S.M.; Uddin, M.E. Bioinformatics Approaches for Structural and Functional Annotation of an Uncharacterized Protein of Helicobacter pylori. Eng. Proc. 2023, 37, 61. [Google Scholar] [CrossRef]
- Kyte, J.; Doolittle, R.F. A simple method for displaying the hydropathic character of a protein. J. Mol. Biol. 1982, 157, 105–132. [Google Scholar] [CrossRef]
- Yousuf, M.; Saikat, A.S.M.; Uddin, M.E. A Bioinformatic Approach for Molecular Characterization and Functional Annotation of an Uncharacterized Protein from Vibrio cholerae. Eng. Proc. 2023, 37, 62. [Google Scholar] [CrossRef]
- Soleymani, F.; Paquet, E.; Viktor, H.; Michalowski, W.; Spinello, D. Protein-protein interaction prediction with deep learning: A comprehensive review. Comput. Struct. Biotechnol. J. 2022, 20, 5316–5341. [Google Scholar] [CrossRef] [PubMed]
- Saikat, A.S.M.; Al-Khafaji, K.; Akter, H.; Choi, J.-G.; Hasan, M.; Lee, S.-S. Nature-Derived Compounds as Potential Bioactive Leads against CDK9-Induced Cancer: Computational and Network Pharmacology Approaches. Processes 2022, 10, 2512. [Google Scholar] [CrossRef]
- Lorenzini, E.; Singer, A.; Singh, B.; Lam, R.; Skarina, T.; Chirgadze, N.Y.; Savchenko, A.; Gupta, R.S. Structure and protein-protein interaction studies on Chlamydia trachomatis protein CT670 (YscO Homolog). J. Bacteriol. 2010, 192, 2746–2756. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wardah, W.; Khan, M.; Sharma, A.; Rashid, M.A. Protein secondary structure prediction using neural networks and deep learning: A review. Comput. Biol. Chem. 2019, 81, 1–8. [Google Scholar] [CrossRef]
- Al Asad, M.; Shorna, S.A.; Saikat, A.S.M.; Uddin, E. Computational Approaches for Structure-Based Functional Annotation of an Uncharacterized Conserved Protein of Acinetobacter baumannii. Eng. Proc. 2023, 37, 25. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).