Synthesis and Evaluation of Thiomethyl-Substituted (4Z)-4-[(Pyrazol-4-yl)methylene]pyrazolone as an Optical Chemosensor †
Abstract
:1. Introduction
2. Materials and Methods
2.1. General
2.2. Synthesis of (4Z)-4-[(5-Hydroxy-3-methyl-1-phenyl-1H-pyrazol-4-yl)(4-thiomethylphenyl)methylene]-5-methyl-2-phenyl-2,4-dihydro-3H-pyrazol-3-one (3)
2.3. Preliminary Chemosensory Studies
3. Results and Discussion
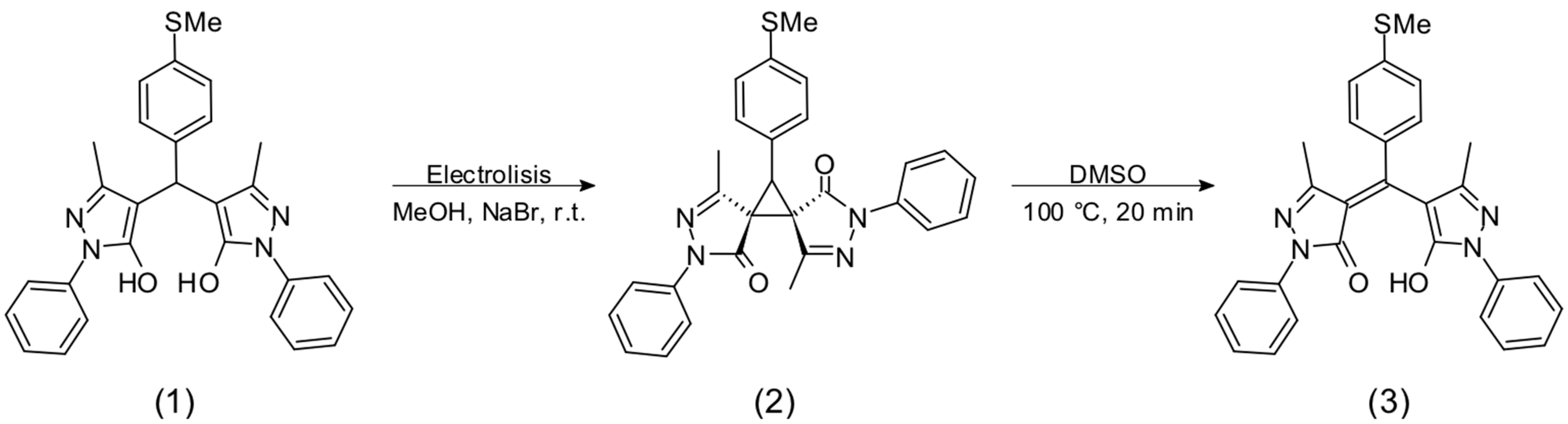
3.1. Synthesis
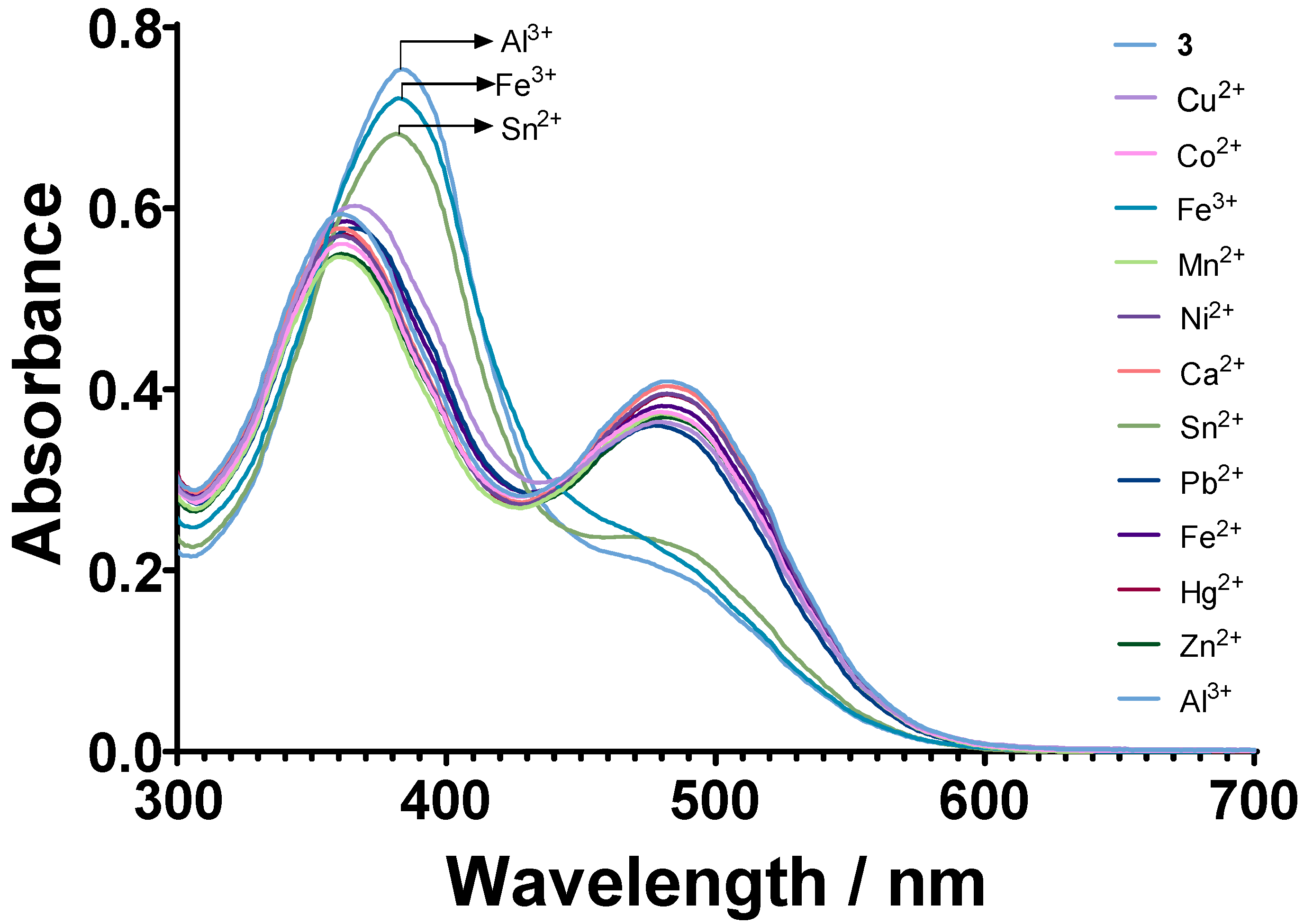
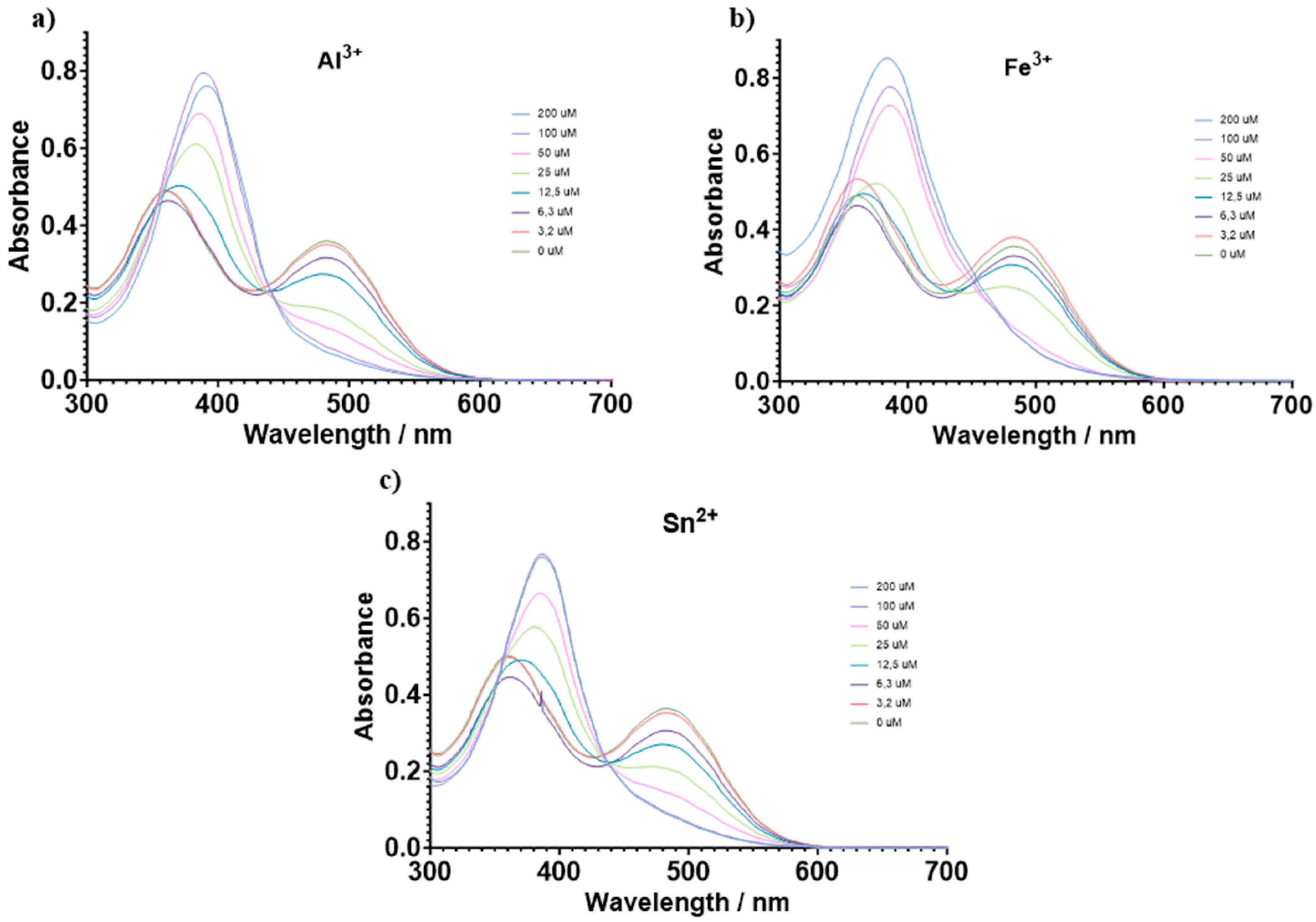
3.2. Chemosensory Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moustakas, M. The Role of Metal Ions in Biology, Biochemistry and Medicine. Materials 2021, 14, 549. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Sharma, R.K.; Gaur, K.; Cátala Torres, J.F.; Loza-Rosas, S.A.; Torres, A.; Saxena, M.; Julin, M.; Tinoco, A.D. Fueling a Hot Debate on the Application of TiO2 Nanoparticles in Sunscreen. Materials 2019, 12, 2317. [Google Scholar] [CrossRef] [PubMed]
- Sperdouli, I.; Moustaka, J.; Antonoglou, O.; Adamakis, I.-D.S.; Dendrinou-Samara, C.; Moustakas, M. Leaf Age-Dependent Effects of Foliar-Sprayed CuZn Nanoparticles on Photosynthetic Efficiency and ROS Generation in Arabidopsis thaliana. Materials 2019, 12, 2498. [Google Scholar] [CrossRef] [PubMed]
- Goharian, A.; Abdullah, M.R. Bioinert Metals (Stainless Steel, Titanium, Cobalt Chromium). In Trauma Plating Systems: Biomechanical, Material, Biological, and Clinical Aspects; Elsevier: Amsterdam, The Netherlands, 2017; pp. 115–142. [Google Scholar] [CrossRef]
- Shetty, R.; Vidya, C.S.N.; Prakash, N.B.; Lux, A.; Vaculík, M. Aluminum Toxicity in Plants and Its Possible Mitigation in Acid Soils by Biochar: A Review. Sci. Total Environ. 2021, 765, 142744. [Google Scholar] [CrossRef] [PubMed]
- Rahimzadeh, M.R.; Rahimzadeh, M.R.; Kazemi, S.; Amiri, R.J.; Pirzadeh, M.; Moghadamnia, A.A. Aluminum Poisoning with Emphasis on Its Mechanism and Treatment of Intoxication. Emerg. Med. Int. 2022, 2022, 1480553. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Song, L.; Han, Y.; Wang, Y.; Tang, X.; Cui, G.; Xu, Z. Effects of Fe3+ on Acute Toxicity and Regeneration of Planarian (Dugesia Japonica) at Different Temperatures. Biomed. Res. Int. 2019, 2019, 8591631. [Google Scholar] [CrossRef] [PubMed]
- Rathinam, B.; Liu, B.T.; Nagarajan, D.; Kaliyamoorthy, S. Systematic Analysis of Colorimetric and Fluorescent Sensors for the Detection of Tin Ions. J. Taiwan Inst. Chem. Eng. 2023, 147, 104909. [Google Scholar] [CrossRef]
- Ibrahim, S.A.; Ragab, A.; El-Ghamry, H.A. Coordination Compounds of Pyrazolone-Based Ligand: Design, Characterization, Biological Evaluation, Antitumor Efficiency, and DNA Binding Evaluation Supported by in Silico Studies. Appl. Organomet. Chem. 2022, 36, e6508. [Google Scholar] [CrossRef]
- Ghorbani, S.; Parnian, R.; Soleimani, E. Pd Nanoparticles Supported on Pyrazolone-Functionalized Hollow Mesoporous Silica as an Excellent Heterogeneous Nanocatalyst for the Selective Oxidation of Benzyl Alcohol. J. Organomet. Chem. 2021, 952, 122025. [Google Scholar] [CrossRef]
- Branković, J.; Milovanović, V.M.; Petrović, Z.D.; Simijonović, D.; Petrović, V.P. Pyrazolone-Type Compounds (Part II): In Vitro and in Silico Evaluation of Antioxidant Potential; Structure-Activity Relationship. RSC Adv. 2023, 13, 2884–2895. [Google Scholar] [CrossRef] [PubMed]
- Cong, S.; Shi, Y.; Yu, G.; Zhong, F.; Li, J.; Liu, J.; Ye, C.; Tan, Z.; Deng, Y. Discovery of Novel 5-(2-Hydroxyphenyl)-2-Phthalide-3(3H)-Pyrazolones as Balanced Multifunctional Agents against Alzheimer’s Disease. Eur. J. Med. Chem. 2023, 250, 115216. [Google Scholar] [CrossRef] [PubMed]
- Kshatriya, R.; Shelke, P.; Mali, S.; Yashwantrao, G.; Pratap, A.; Saha, S. Synthesis and Evaluation of Anticancer Activity of Pyrazolone Appended Triarylmethanes (TRAMs). ChemistrySelect 2021, 6, 6230–6239. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, L.; Wang, X.; Zhao, Y.; Chen, X. Cu(II) and Zn(II) Crystal Complexes Based on Pyrazolone: Synthesis and Application as Antibacterial Agents. Inorganica Chim. Acta 2023, 556, 121618. [Google Scholar] [CrossRef]
- Tigreros, A.; Portilla, J. Recent Progress in Chemosensors Based on Pyrazole Derivatives. RSC Adv. 2020, 10, 19693. [Google Scholar] [CrossRef] [PubMed]
- Kaliraj, K.; Xia, L.; Edison, T.N.J.I.; Lee, Y.R. Straightforward synthesis of diverse dipyrazolylmethane derivatives and their application for fluorescence sensing of Cu2+ ions. RSC Adv. 2016, 6, 56323–56329. [Google Scholar] [CrossRef]
- Cadena-Cruz, J.E.; Guamán-Ortiz, L.M.; Romero-Benavides, J.C.; Bailon-Moscoso, N.; Murillo-Sotomayor, K.E.; Ortiz-Guamán, N.V.; Heredia-Moya, J. Synthesis of 4,4′-(arylmethylene)bis(3-methyl-1-phenyl-1H-pyrazol-5-ols) and evaluation of their antioxidant and anticancer activities. BMC Chem. 2021, 15, 38. [Google Scholar] [CrossRef] [PubMed]
- Barreiro-Costa, O.; Morales-Noboa, G.; Rojas-Silva, P.; Lara-Barba, E.; Santamaría-Aguirre, J.; Bailón-Moscoso, N.; Romero-Benavides, J.C.; Herrera, A.; Cueva, C.; Ron-Garrido, L.; et al. Synthesis and evaluation of biological activities of bis(spiropyrazolone)cyclopropanes: A potential application against leishmaniasis. Molecules 2021, 26, 4960. [Google Scholar] [CrossRef] [PubMed]
- Dorofeeva, E.; Elinson, M.; Vereshchagin, A.; Nigmatov, A.; Bushmarinov, I.; Nikishin, G. Stereoselective Thermal Isomerization of Bis(spiropyrazolone)cyclopropanes into (4Z)-4-[(Pyrazol-4-yl)methylene]pyrazolones. Synlett 2013, 24, 827–830. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazón Ayala, P.V.; Romero-Benavides, J.C.; Heredia-Moya, J. Synthesis and Evaluation of Thiomethyl-Substituted (4Z)-4-[(Pyrazol-4-yl)methylene]pyrazolone as an Optical Chemosensor. Chem. Proc. 2023, 14, 50. https://doi.org/10.3390/ecsoc-27-16123
Mazón Ayala PV, Romero-Benavides JC, Heredia-Moya J. Synthesis and Evaluation of Thiomethyl-Substituted (4Z)-4-[(Pyrazol-4-yl)methylene]pyrazolone as an Optical Chemosensor. Chemistry Proceedings. 2023; 14(1):50. https://doi.org/10.3390/ecsoc-27-16123
Chicago/Turabian StyleMazón Ayala, Paola V., Juan Carlos Romero-Benavides, and Jorge Heredia-Moya. 2023. "Synthesis and Evaluation of Thiomethyl-Substituted (4Z)-4-[(Pyrazol-4-yl)methylene]pyrazolone as an Optical Chemosensor" Chemistry Proceedings 14, no. 1: 50. https://doi.org/10.3390/ecsoc-27-16123
APA StyleMazón Ayala, P. V., Romero-Benavides, J. C., & Heredia-Moya, J. (2023). Synthesis and Evaluation of Thiomethyl-Substituted (4Z)-4-[(Pyrazol-4-yl)methylene]pyrazolone as an Optical Chemosensor. Chemistry Proceedings, 14(1), 50. https://doi.org/10.3390/ecsoc-27-16123






