Transesterification of a Natural Epoxythymol Is Favored under Alkaline Conditions, Preserving the Enantiomeric Purity †
Abstract
:1. Introduction
2. Material and Methods
2.1. Experimental Section
2.2. Plant Material
2.3. Extraction and Isolation
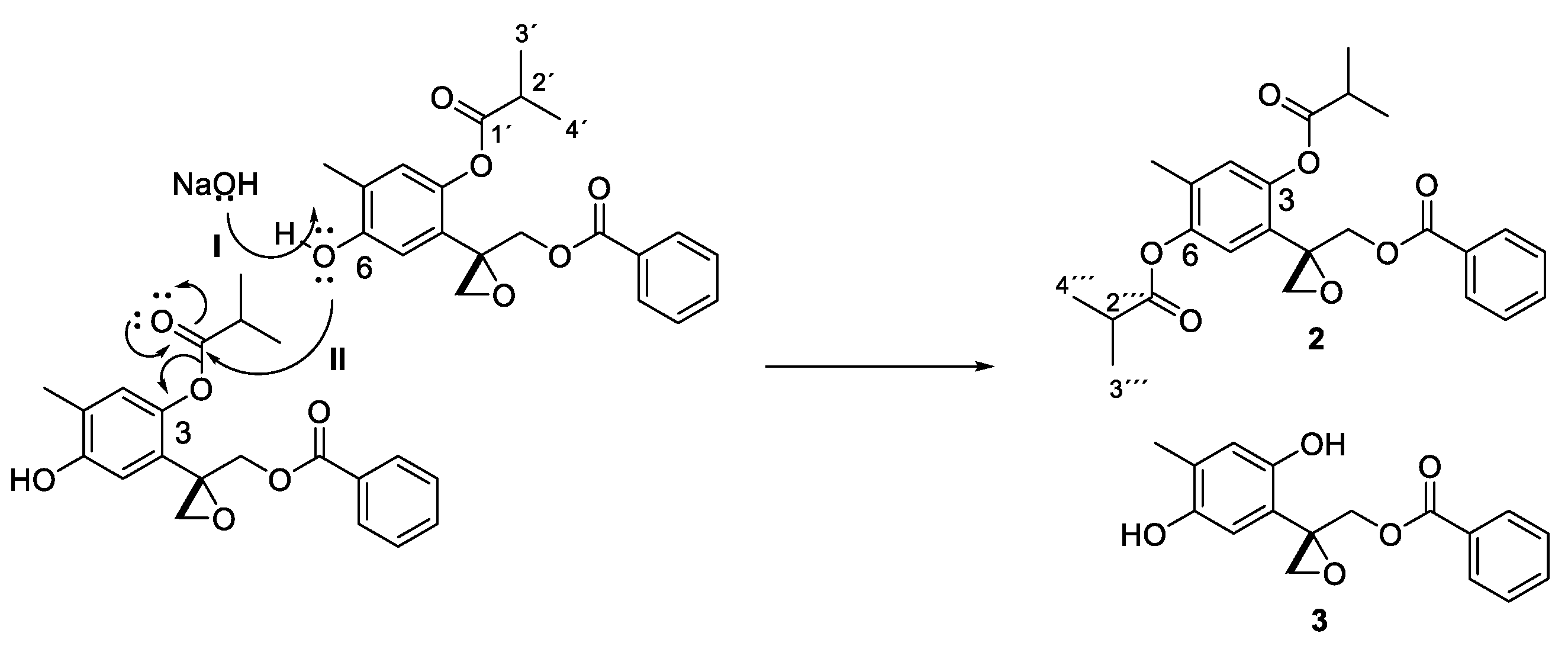
2.4. Transesterification Reaction under Basic Conditions
2.5. Epimerization Reaction of Epoxythymol 1
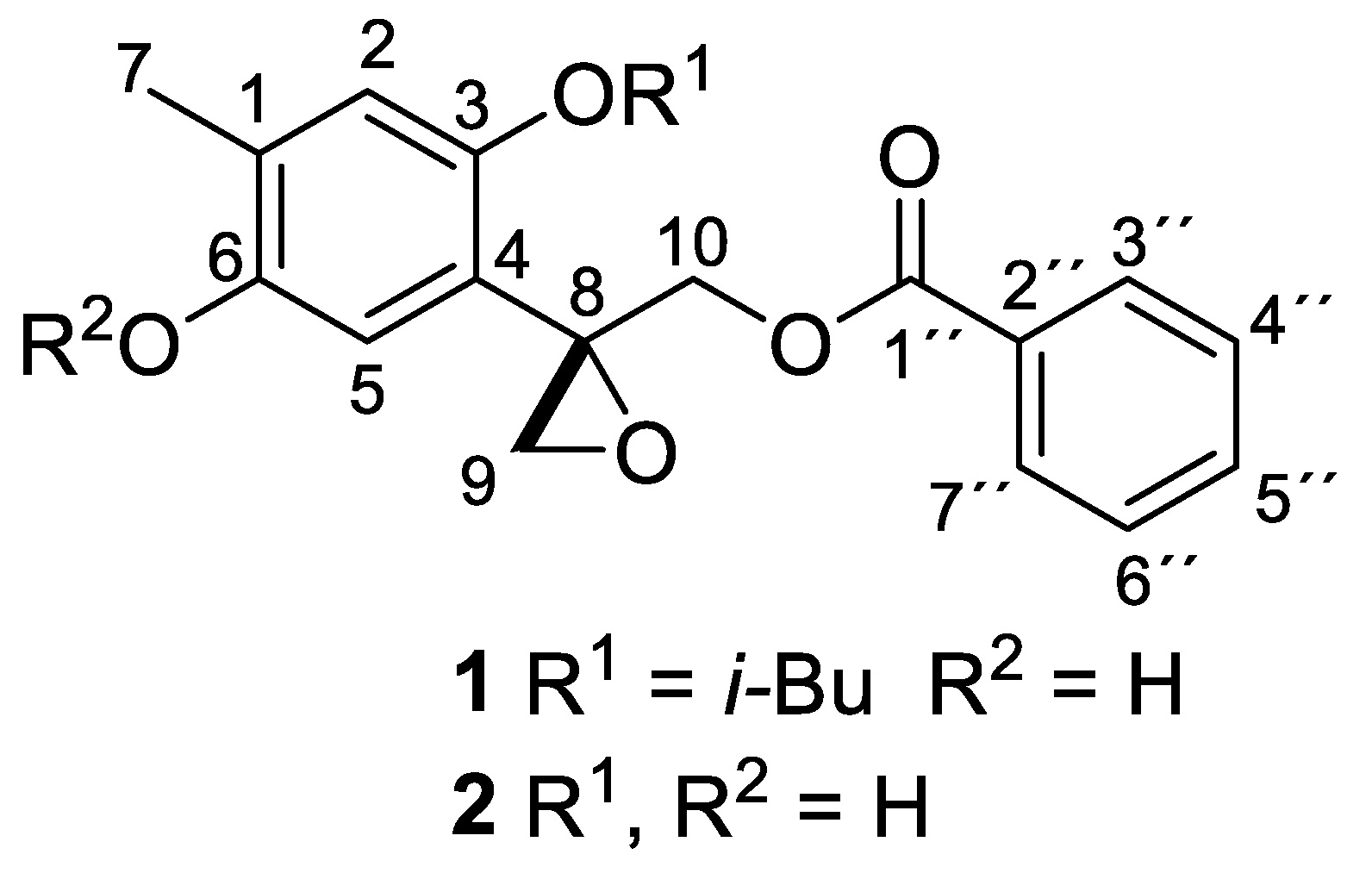
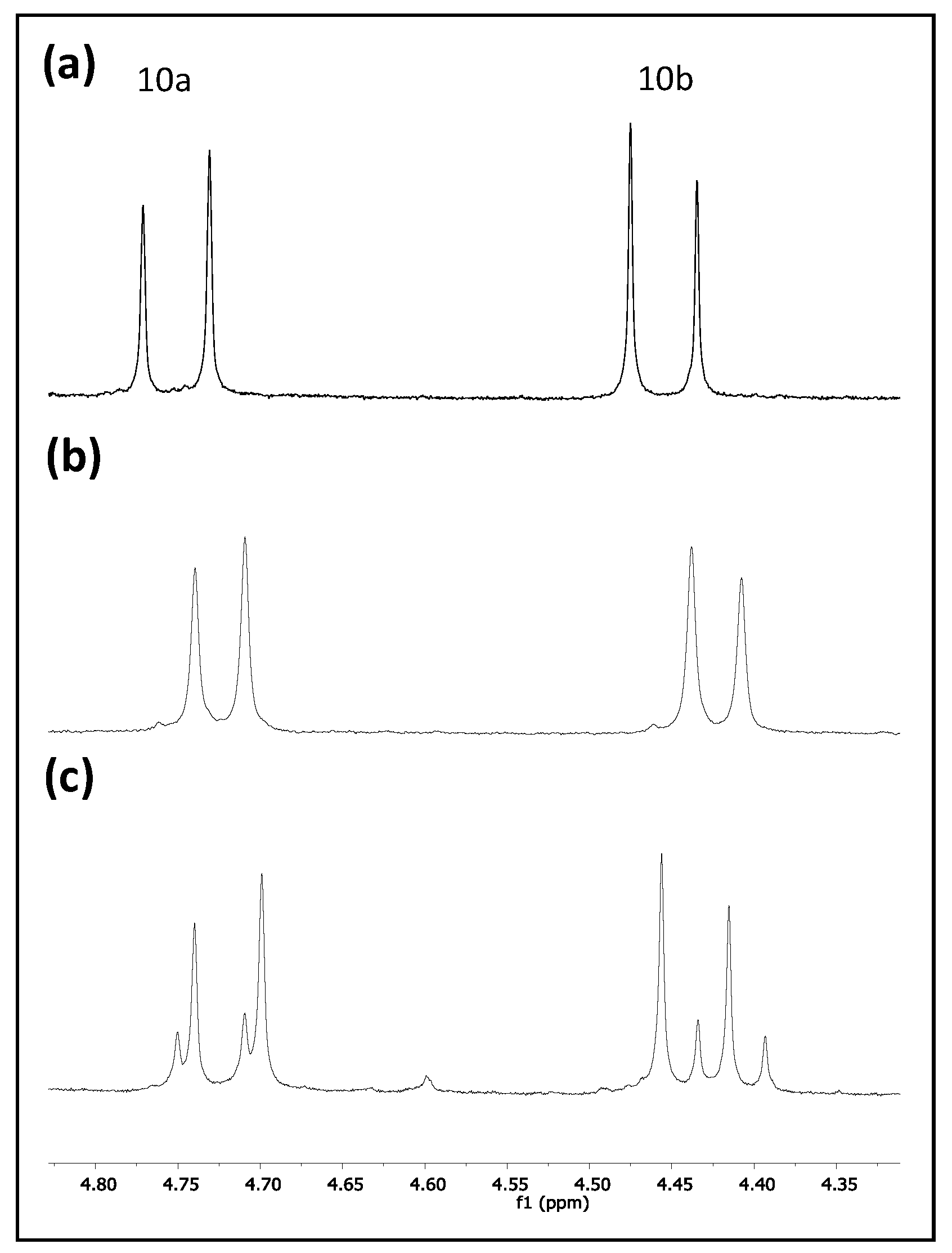
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Otera, J. Transesterification. Chem. Rev. 1993, 93, 1449–1470. [Google Scholar] [CrossRef]
- Bohlmann, F.; Niedballa, U.; Schulz, J. Über einige Thymolderivate aus Gaillardia- und Helenium-Arten. Chem. Berichte 1969, 102, 864–871. [Google Scholar] [CrossRef]
- Tori, M.; Ohara, Y.; Nakashima, K.; Sono, M. Thymol Derivatives from Eupatorium fortunei. J. Nat. Prod. 2001, 64, 1048–1051. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.-X.; Li, Y.; Pan, J.; Gao, K. Terpenoids from Eupatorium fortunei Turcz. Helv. Chim. Acta 2006, 89, 558–566. [Google Scholar] [CrossRef]
- Talavera-Alemán, A.; Rodríguez-García, G.; López, Y.; García-Gutiérrez, H.A.; Torres-Valencia, J.M.; del Río, R.E.; Cerda-García-Rojas, C.M.; Joseph-Nathan, P.; Gómez-Hurtado, M.A. Systematic Evaluation of Thymol Derivatives Possessing Stereogenic or Prostereogenic Centers. Phytochem. Rev. 2016, 15, 251–277. [Google Scholar] [CrossRef]
- Boukhris, M.; Bouaziz, M.; Feki, I.; Jemai, H.; El Feki, A.; Sayadi, S. Hypoglycemic and Antioxidant Effects of Leaf Essential Oil of Pelargonium graveolens L’Hér. in Alloxan Induced Diabetic Rats. Lipids Health Dis. 2012, 11, 81. [Google Scholar] [CrossRef] [PubMed]
- Oz, M.; Lozon, Y.; Sultan, A.; Yang, K.-H.S.; Galadari, S. Effects of Monoterpenes on Ion Channels of Excitable Cells. Pharmacol. Ther. 2015, 152, 83–97. [Google Scholar] [CrossRef] [PubMed]
- Akerele, O. Las Plantas Medicinales: Un Tesoro Que No Debemos Desperdiciar. Foro Mund. De La Salud 1993, 14, 390–395. [Google Scholar]
- Pérez-Vásquez, A.; Linares, E.; Bye, R.; Cerda-García-Rojas, C.M.; Mata, R. Phytotoxic Activity and Conformational Analysis of Thymol Analogs from Hofmeisteria schaffneri. Phytochemistry 2008, 69, 1339–1347. [Google Scholar] [CrossRef]
- Liang, H.; Bao, F.; Dong, X.; Tan, R.; Zhang, C.; Lu, Q.; Cheng, Y. Antibacterial Thymol Derivatives Isolated from Centipeda minima. Molecules 2007, 12, 1606–1613. [Google Scholar] [CrossRef]
- Bustos-Brito, C.; Sánchez-Castellanos, M.; Esquivel, B.; Calderón, J.S.; Calzada, F.; Yepez-Mulia, L.; Hernández-Barragán, A.; Joseph-Nathan, P.; Cuevas, G.; Quijano, L. Structure, Absolute Configuration, and Antidiarrheal Activity of a Thymol Derivative from Ageratina cylindrica. J. Nat. Prod. 2014, 77, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Reist, M.; Testa, B.; Carrupt, P.-A.; Jung, M.; Schurig, V. Racemization, Enantiomerization, Diastereomerization, and Epimerization: Their Meaning and Pharmacological Significance. Chirality 1995, 7, 396–400. [Google Scholar] [CrossRef]
- Arreaga-González, H.M.; Pardo-Novoa, J.C.; del Río, R.E.; Rodríguez-García, G.; Torres-Valencia, J.M.; Manríquez-Torres, J.J.; Cerda-García-Rojas, C.M.; Joseph-Nathan, P.; Gómez-Hurtado, M.A. Methodology for the Absolute Configuration Determination of Epoxythymols Using the Constituents of Ageratina glabrata. J. Nat. Prod. 2018, 81, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Bustos-Brito, C.; Vázquez-Heredia, V.J.; Calzada, F.; Yépez-Mulia, L.; Calderón, J.S.; Hernández-Ortega, S.; Esquivel, B.; García-Hernández, N.; Quijano, L. Antidiarrheal Thymol Derivatives from Ageratina glabrata. Structure and Absolute Configuration of 10-Benzoyloxy-8,9-epoxy-6-hydroxythymol Isobutyrate. Molecules 2016, 21, 1132. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lorenzo-García, J.M.; Oliveros-Ortiz, A.J.; Arreaga-González, H.M.; Cortés-García, C.J.; del Río, R.E.; Rodríguez-García, G.; Gómez-Hurtado, M.A. Transesterification of a Natural Epoxythymol Is Favored under Alkaline Conditions, Preserving the Enantiomeric Purity. Chem. Proc. 2023, 14, 30. https://doi.org/10.3390/ecsoc-27-16140
Lorenzo-García JM, Oliveros-Ortiz AJ, Arreaga-González HM, Cortés-García CJ, del Río RE, Rodríguez-García G, Gómez-Hurtado MA. Transesterification of a Natural Epoxythymol Is Favored under Alkaline Conditions, Preserving the Enantiomeric Purity. Chemistry Proceedings. 2023; 14(1):30. https://doi.org/10.3390/ecsoc-27-16140
Chicago/Turabian StyleLorenzo-García, Jessica M., Antonio J. Oliveros-Ortiz, Héctor M. Arreaga-González, Carlos J. Cortés-García, Rosa E. del Río, Gabriela Rodríguez-García, and Mario A. Gómez-Hurtado. 2023. "Transesterification of a Natural Epoxythymol Is Favored under Alkaline Conditions, Preserving the Enantiomeric Purity" Chemistry Proceedings 14, no. 1: 30. https://doi.org/10.3390/ecsoc-27-16140
APA StyleLorenzo-García, J. M., Oliveros-Ortiz, A. J., Arreaga-González, H. M., Cortés-García, C. J., del Río, R. E., Rodríguez-García, G., & Gómez-Hurtado, M. A. (2023). Transesterification of a Natural Epoxythymol Is Favored under Alkaline Conditions, Preserving the Enantiomeric Purity. Chemistry Proceedings, 14(1), 30. https://doi.org/10.3390/ecsoc-27-16140





