Pb(II) Adsorption by a Calcium Metal-Organic Framework †
Abstract
:1. Introduction
2. Experimental Method
2.1. Synthesis of Experimental [Ca(H2btec)·H2O]n
2.2. Pb(II) Ions Adsorption
2.3. Results and Discussion
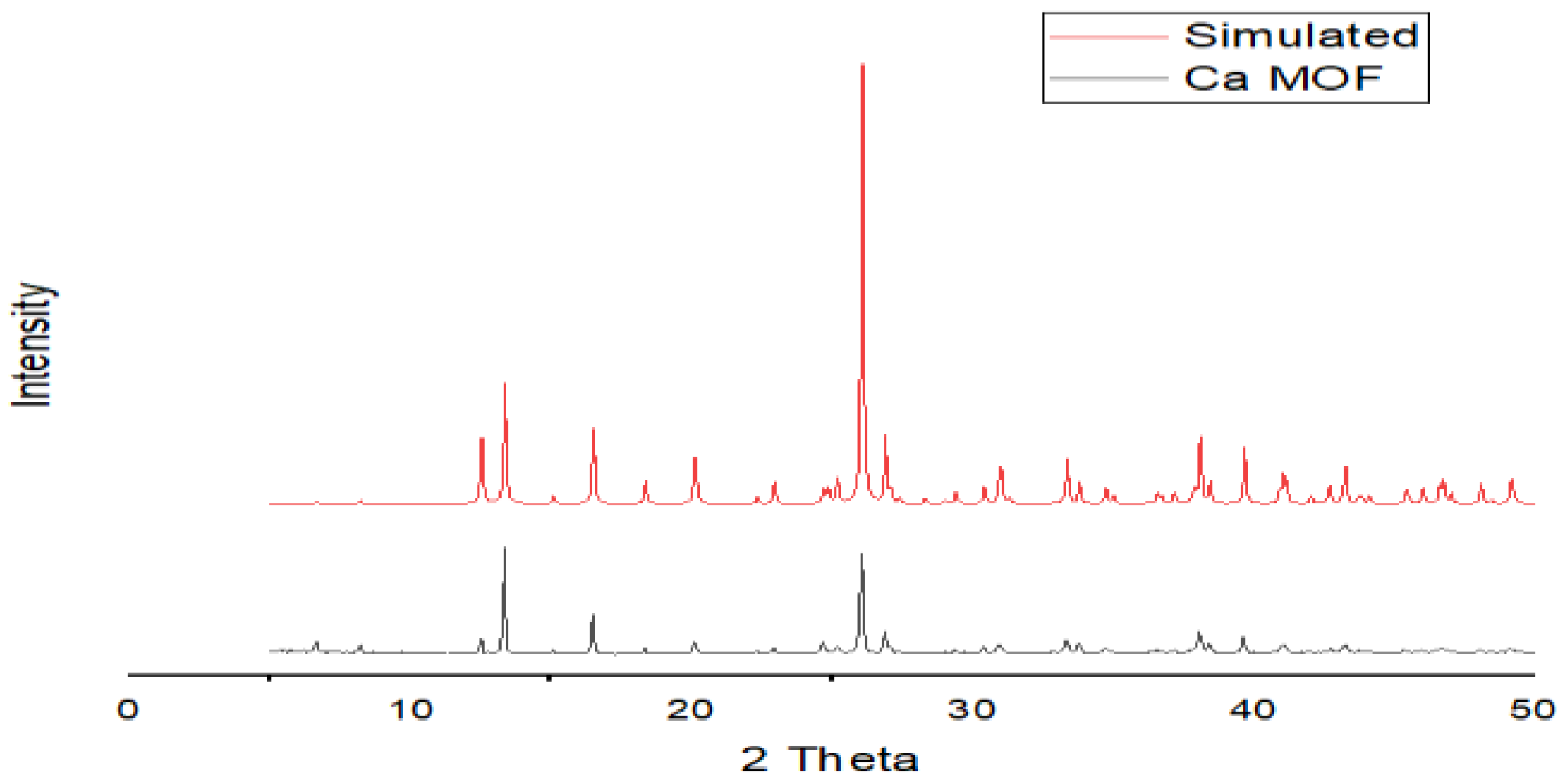
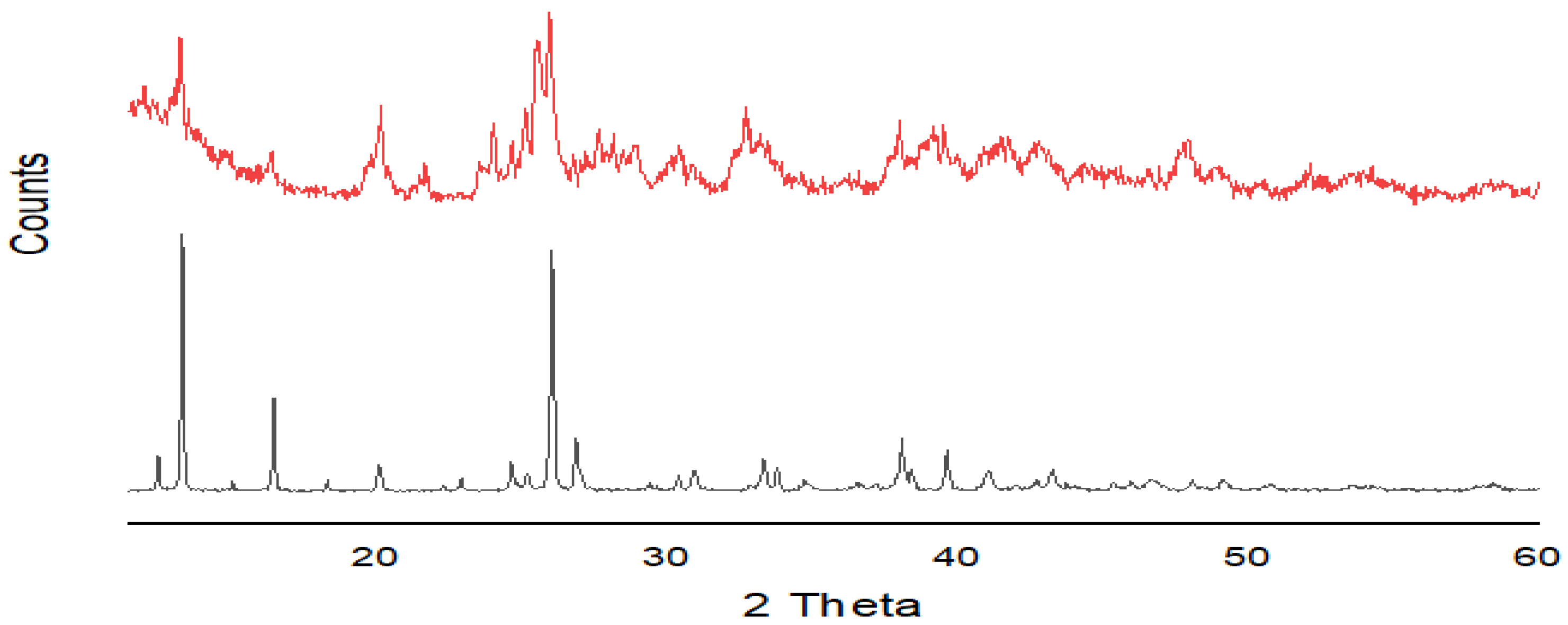
2.3.1. XRD Pattern
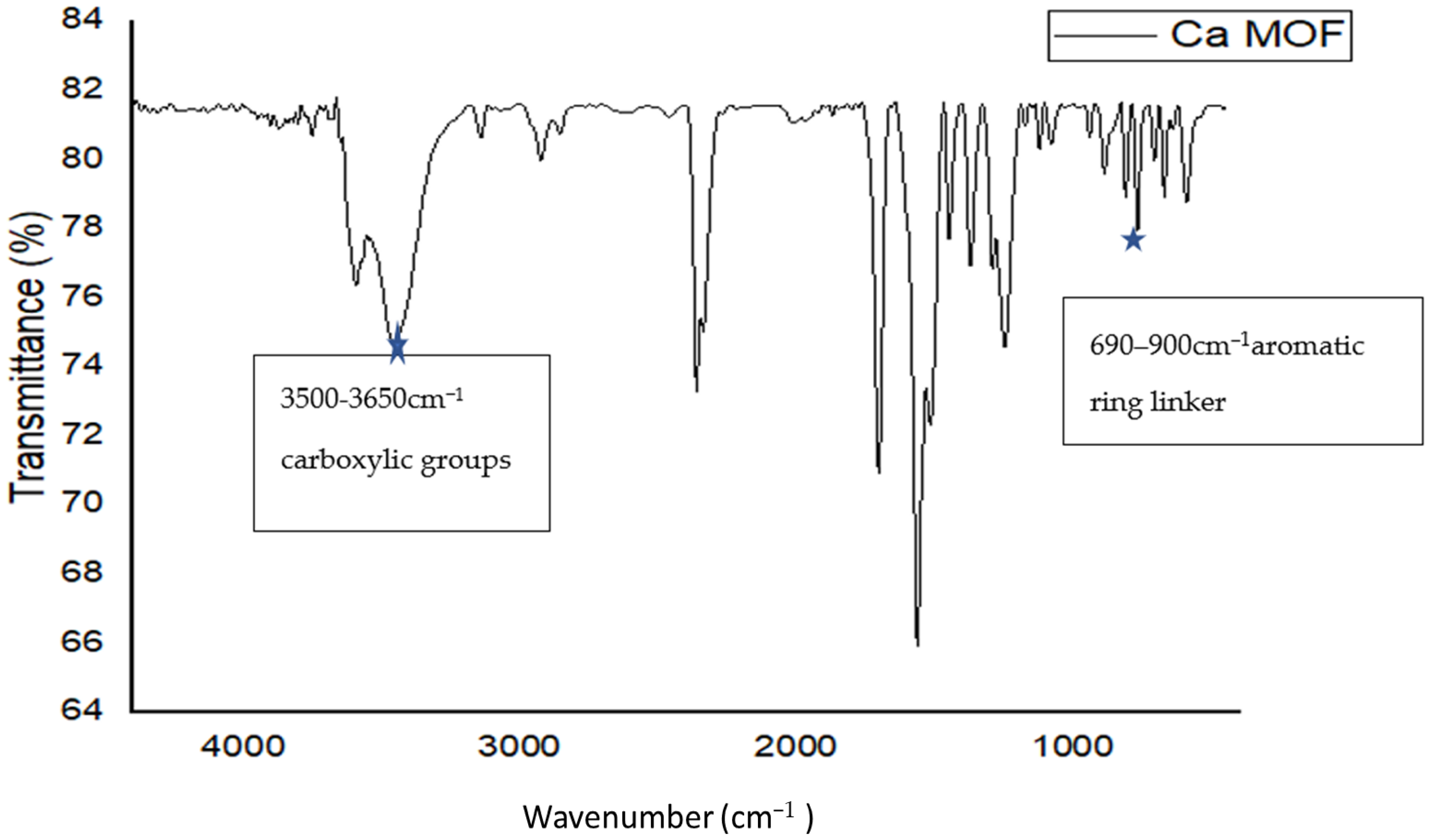
2.3.2. FTIR Spectrum
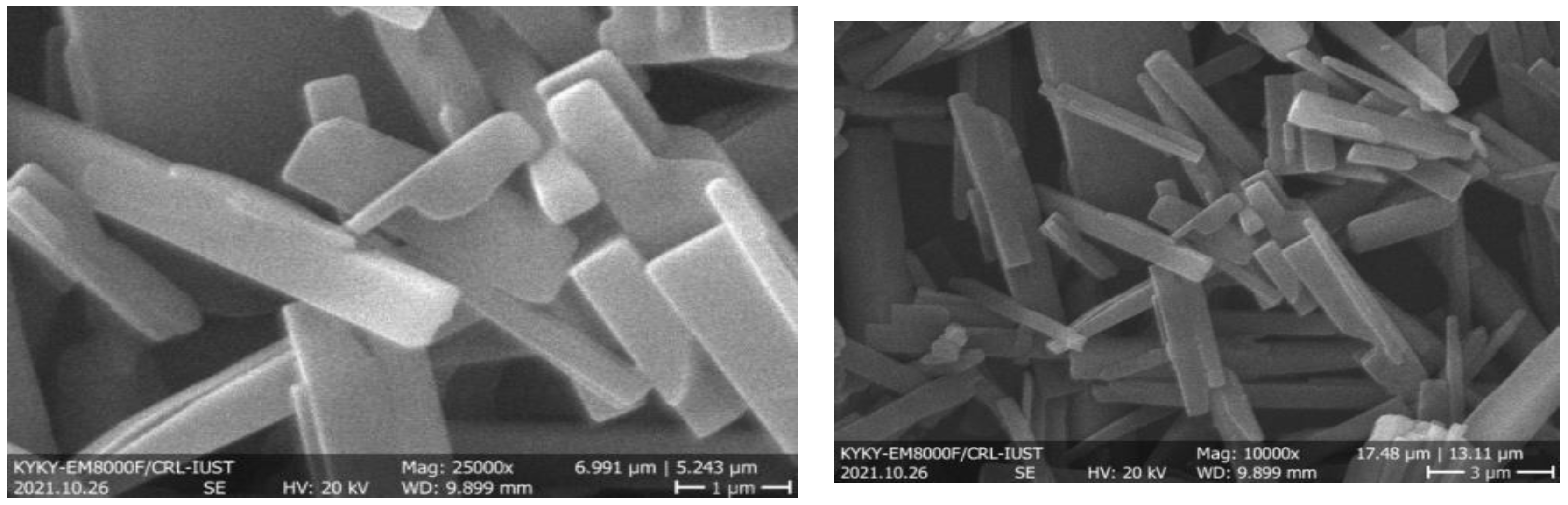
2.3.3. Scanning Electron Microscopy
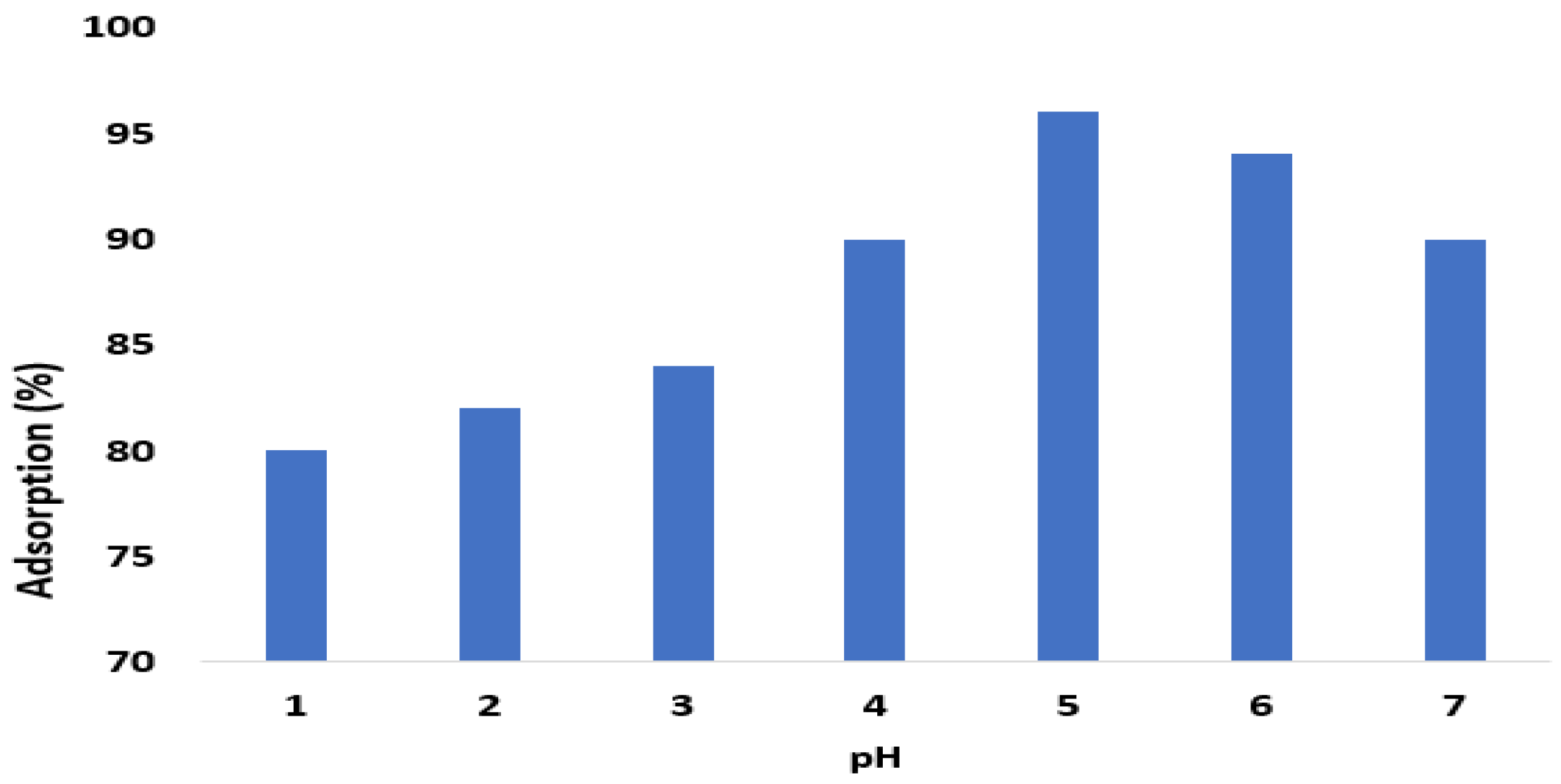
2.3.4. Effect of pH and Time on Adsorption
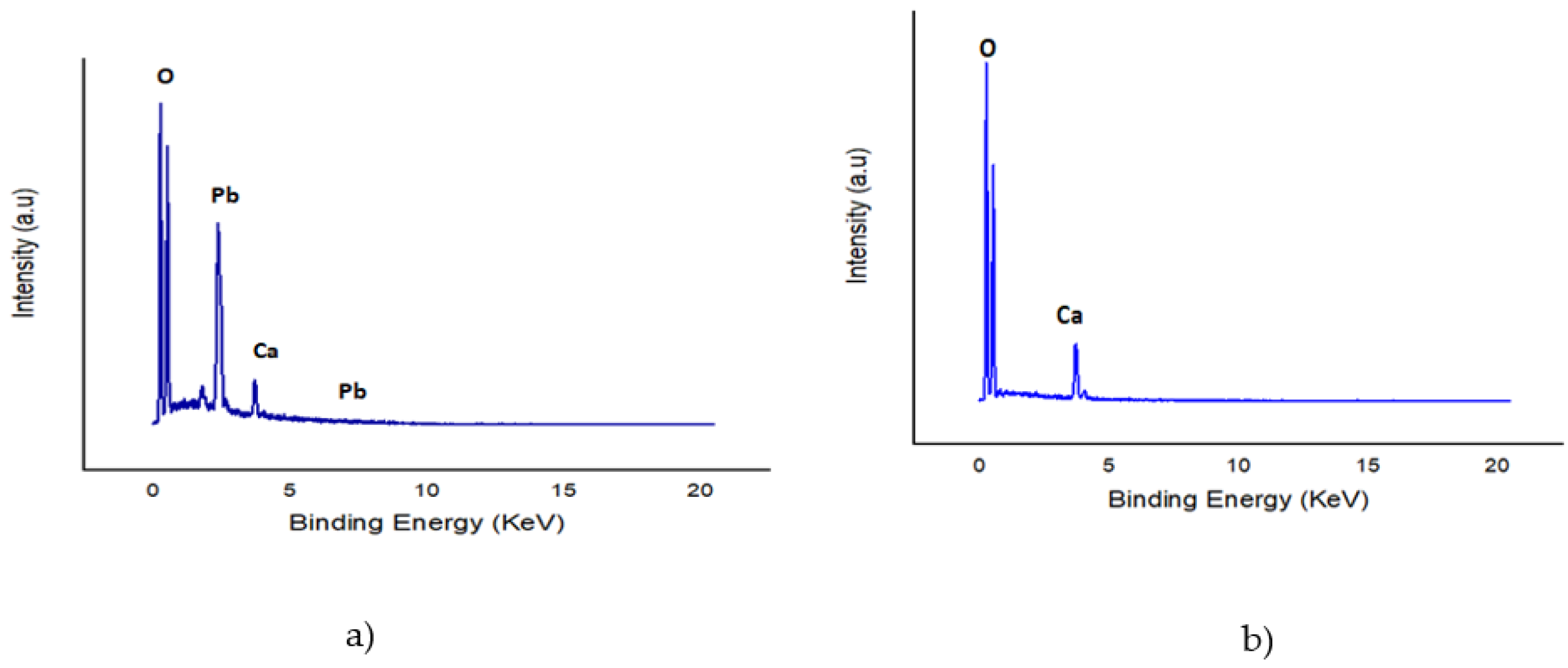
2.3.5. Adsorption of Metallic Ions
2.3.6. Desorption after Adsorption
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rouhani, F.; Morsali, A. Fast and Selective Heavy Metal Removal by a Novel Metal-Organic Framework Designed with In-Situ Ligand Building Block Fabrication Bearing Free Nitrogen. Chem. A Eur. J. 2018, 24, 5529–5537. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Yuan, J.; Tan, X.; Zhang, W.; Fang, M.; Wang, X. Efficient removal of Pb2+ by Tb-MOFs: Identifying the adsorption mechanism through experimental and theoretical investigations. Environ. Sci. Nano 2019, 6, 261–272. [Google Scholar] [CrossRef]
- Du, S.; Ji, C.; Xin, X.; Zhuang, M.; Yu, X.; Lu, J.; Lu, Y.; Sun, D. Syntheses, structures and characteristics of four alkaline-earth metal-organic frameworks (MOFs) based on benzene-1,2,4,5-tetracarboxylicacid and its derivative ligand. J. Mol. Struct. 2017, 1130, 565–572. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aghaee, M.; Manteghi, F. Pb(II) Adsorption by a Calcium Metal-Organic Framework. Chem. Proc. 2022, 12, 74. https://doi.org/10.3390/ecsoc-26-13723
Aghaee M, Manteghi F. Pb(II) Adsorption by a Calcium Metal-Organic Framework. Chemistry Proceedings. 2022; 12(1):74. https://doi.org/10.3390/ecsoc-26-13723
Chicago/Turabian StyleAghaee, Mina, and Faranak Manteghi. 2022. "Pb(II) Adsorption by a Calcium Metal-Organic Framework" Chemistry Proceedings 12, no. 1: 74. https://doi.org/10.3390/ecsoc-26-13723
APA StyleAghaee, M., & Manteghi, F. (2022). Pb(II) Adsorption by a Calcium Metal-Organic Framework. Chemistry Proceedings, 12(1), 74. https://doi.org/10.3390/ecsoc-26-13723




