Abstract
A transnational concern for the healthy development of human beings is antimicrobial resistance (AMR). The rising rates of microbial resistance present a serious issue for the development of human life and hence it is essential to find and create newer antimicrobial drugs with unique modes of action. One approach used these days to solving this challenge is the use of heterocyclics to create hybrid compounds by fusing two or more bioactive heterocyclic moieties into a single molecular platform. This study discusses the many hybrid approaches that have been used to produce possible novel antimicrobial medicines that are both safe and effective. The landscaping of heterocycles such as thiazole derivatives is covered in the current review paper. In this paper, all the extensive approaches of heterocyclic composites, primarily thiazole derivatives, exhibit vibrant biological activity. The purpose of this work is to support methods that may be used to create various thiazole derivatives and their biological activity. This paper will offer great recommendations for potential medicine designs in the future.
1. Introduction
Thiazole is a five-membered molecule with two hetero atoms—N and S—in the 1 and 3 positions of the ring, placing it in the most significant group of heterocyclics. Both an electron-donating (-S-) and an electron-accepting (-C=N-) group are present in this five-membered heterocyclic nucleus, and together they produce a stable heterocyclic molecule. Due to its adaptable building blocks as bioactive chemicals, thiazole is a wonder nucleus. This substance does not exist naturally, although it can be found in a variety of natural products, including cyclopeptides, alkaloids, anabolic steroids, flavones, and vitamin B1-thiamine [1,2,3]. The pharmacophores of the molecules that include thiazole and its derivative nucleus have substantial biological promise [2,3]. These thiazoles and their derivatives all exhibit several biologically significant biological activities, including antibacterial (active against a bacterial infection), antiprotozoal (active against protozoan infection), antitubercular (active against mycobacterium tuberculosis), and antifungal (active against mycoses, such as athlete’s foot, ringworm, candidiasis, and serious systemic infections), anthelmintic [4,5] (against infections of animals with parasitic worms) [6], anti-diuretic, and anti-Alzheimer (effective against amyloid plaques, which are brain lesions linked to Alzheimer’s disease) (active in opposing diuresis) (Figure 1). Additionally, because of their numerous uses in the pharmaceutical industry, all of these thiazole derivatives have garnered a lot of attention.
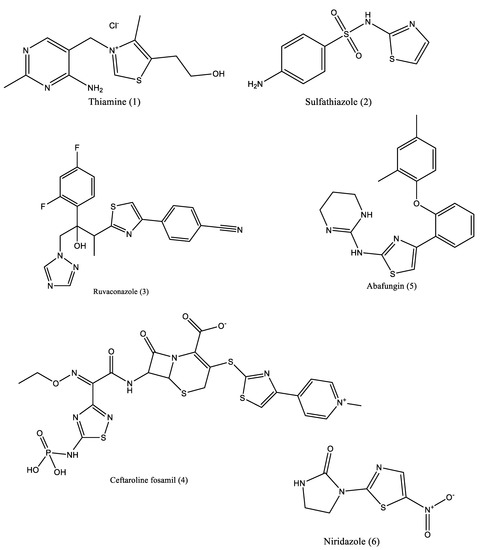
Figure 1.
The structure (1–6) having thiazole moiety and possess substantial biological activities [1,2,3,4,5,6].
A recent study by several researchers revealed the amphiphilic properties of thiazole derivatives. Due to their amphiphilic nature, these derivatives can work against microorganisms and make it easier for them to become entrenched in the cell membranes of microbes such as bacteria and fungi. Figure 2 provides a very clear illustration of the amphiphilic nature of thiazole derivatives. Figure 2 depicts the hydrophobic (which has a high affinity for lipids) and hydrophilic components. This characteristic boosts its capacity to easily permeate into bacterial cell membranes for inhibitory activities [7]. These thiazole compounds are effective against both species of bacteria, i.e., due to their hydrophilic and hydrophobic properties, they may be both effective against Gram-positive and Gram-negative bacteria [8]. The process of embedding in the cell membrane of microbes will lead to the leakage of cytoplasm, cell physiology disturbances, and apoptosis [9].
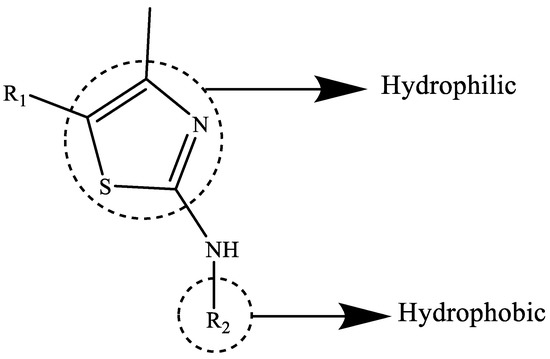
Figure 2.
This figure depicts the amphiphilic character of thiazole derivatives [10,11].
Figure 3 shows the resonance of thiazole structures (delocalization of electrons is displayed in the structure of a molecule if that structure bonding cannot be described by the Lewis formula). The bond order of the p orbital is represented in these resonating forms of thiazoles using a variety of molecular orbital approaches. These distinctive molecular orbital approaches list the several ways that this thiazole molecule is aromatic with diene-like characteristics [12]. Thiazole’s structure adheres to Huckel’s rule of aromaticity, which is characterized by the delocalization of electrons and correlates to sulfur’s loan pair of electrons-based Huckel rule [13]. Thiazole derivatives are sought-after model substances for chemical research because of their planar and aromatic structures, which display greater electron density [14].
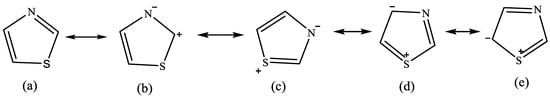
Figure 3.
The thiazole resonating structures are shown in this illustration. The Subfigures (a–e) showed movement of electron and displayed that the molecule is aromatic with some diene character. [15].
The method employed to characterize the aromatic behavior of this heterocyclic ring was verified using 1H NMR spectroscopy since thiazole adheres to the Huckel rule. The chemical shift (the frequency of an atomic nucleus that is resonant in relation to a standard in a magnetic field) of the protons in this molecule was reported to be between 7.27 and 8.77 ppm [16]. According to several published investigations, the replacement of different substituents at various positions of the carbon atoms, i.e., at positions 2, 4, and 5 slowed down the pace of the reaction of thiazole and its numerous derivatives, which may warrant additional structural thought [17]. The influence of various groups, such as electron-donating or withdrawing substituents, was recognized when they were present in any position of the thiazole ring and its derivatives, which serves as a better example of the replacement of various functional groups at the carbon position. Because of these groups, the molecule’s basicity or acidity will increase.
Now let’s discuss another highly potent thiazole derivative, thiazolidinone, which is a physiologically significant heterocyclic ring with sulfur at position 1, nitrogen at position 3, and a carbonyl group at either position 2, 4, or 5 (Figure 4).
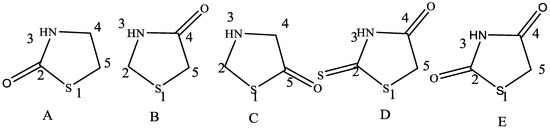
Figure 4.
This figure depicts the structures of Thiazolidinone. The subfigures (A–E) examples of derivatives of thiazolidinone that have variable activity based on the substitution at different position of ring [17].
In Figure 4, 2-thiazolidinone (A), 4-thiazolidinone (B), 5-thiazolidinone (C), 2-thioxo-4-thiazolidinone (D), and thiazolidine-2,4-dione (E) are examples of derivatives of thiazolidinone that have variable activity based on the substitution at different locations. The BRD4 bromodomain, which predicts the behavior of histone proteins and controls the conversion of cell DNA to RNA, has recently been identified as 2-thiazolidinone’s most significant pharmacological effect. The inhibitors 5-thiazolidinone and 2-thioxo-4-thiazolidinone (commonly known as rhodanine) are essential scaffolds of several drug-like compounds, and it further stimulates the growth and proliferation of tumor cells. Thiazolidine-2,4-dione (TZD), a clinically utilized medication to treat type 2 diabetes, has a powerful hypoglycemia ingredient [1,2,3,4,5,6,7,14,15]. A wide range of biological activities, including antitubercular, antibacterial, anti-inflammatory, antiviral, and antidiabetic, have been linked to the 4-thiazolidinone moiety, often known as the “wonder nucleus” or “magic moiety”. In the current review, the literature study was carried out utilizing online research, including database searches in Springer, Google Scholar, Science Direct, PubMed, and SCOPUS between 2000 and 2022. The broad methods of heterocyclic composites, especially thiazole derivatives, that demonstrate lively biological activity as well as numerous enzymes involved in bacterial growth that are inhibited by 4-thiazolidinones have been discussed in this work.
The purpose of this work is to support the methods that may be used to create various thiazole derivatives and their biological activity. This paper will offer great recommendations for potential medicine designs in the future.
2. Synthesis of Thiazole Derivatives
2.1. General Information
An intriguing area of therapeutic science has been the synthesis of heterocyclic rings. Different heterocyclic compounds of nitrogen and sulfur offer adaptable structural frameworks for medication discovery and design. Numerous synthetic techniques, including one-pot and two-pot processes as well as environmentally friendly synthesis methods, are available to create different thiazolidinone derivatives. The 2, 3, and 5 locations are the active sites, as was already mentioned, and the nucleus exhibits outstanding biological features with even the smallest substitution. The various types of substituted derivatives include dialkyl thiazolidinones, substituted 2-thiono-4 thiazolidinones, substituted 2,3-disubstituted thiazolidine–dineones, substituted 2,4-disubstituted thiazolidinones, and more. Regarding their synthesis, we talk about a couple of them here.
2.2. Synthesis of 2,3-Disubstituted Thiazole
In order to create potent therapeutic agents for the treatment of cancer, Santana et al. [18] reported the synthesis of novel compounds such as (Z)-2-(((E)-4-(trifluoromethyl) benzylidene) hydrazono)-2,3-dihydrothiazole, which belong to thiosemicarbazones and thiazoles. In Figure 5, reaction involving 4-trifluoromethylbenzaldehyde and the corresponding thio-semicarbazide under reflux and a catalytic quantity of HCl, the intermediate thiosemicarbazones were created. These intermediate compounds subsequently combine with various halogenated ketones to produce a range of products with yields ranging from 22% to 94%.
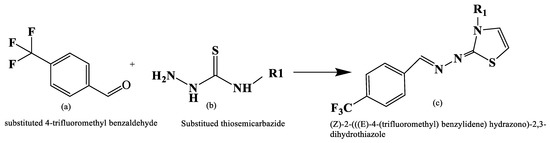
Figure 5.
Synthesis of 2,3-disubstituted thiazole derivatives [18].
2.3. Synthesis of 2,3,4-Trisubstituted Thiazole
The synthesis of substituted thiazoles starting from enamines and elemental sulfur depicted in Figure 6 through to the production of carbon and sulfur bonds was described by Yan et al. [19]. Enamines are created when a secondary amine reacts with an aldehyde or ketone. They are nucleophilic because they have a substantial resonance form in which the alpha carbon carries a negative charge. Enamines interact with electrophiles such as Michael acceptors and alkyl halides to form reactions.
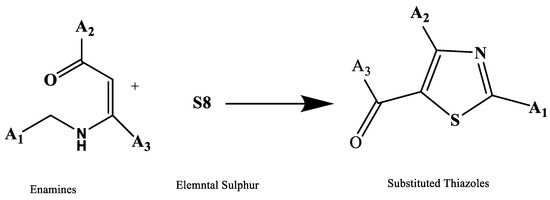
Figure 6.
Synthesis of 2,3-disubstituted thiazole derivatives [19].
2.4. Synthesis of Thiazoyl Derivatives
By reacting 5-acetyl-2-amino-4-methylthiazole with thiocarbohydrazide (CH6N4S) and thiosemicarbazide (CH5N3S) in 100% ethanol and the presence of a catalytic quantity of concentrated HCl, as shown below, Gomha et al. [20] reported the synthesis of novel thiazolyl–thiazole derivatives depicted in Figure 7.
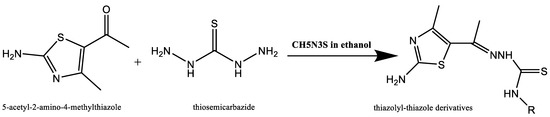
Figure 7.
Synthesis of thiazolyl–thiazole derivatives [20].
2.5. Synthesis of 2,5-Disubstituted Thiazole Derivatives
A copper-catalyzed aerobic three-component annulation shown in the Figure 8 for the synthesis of 2,5-disubstituted thiazole derivatives has recently been revealed by the Jiao group [20]. These substituted amines, substituted benzaldehyde, and elemental sulfur reactions resulted in the formation of these thiazole derivatives in the presence of copper bromide (CuBr2), 1,10-phenanthroline (1,10-Phen).

Figure 8.
Synthesis of 2,5-disubstituted thiazole derivatives [20].
2.6. Synthesis of 2,4,5-Trisubstituted Thiazoles
A modular multi-component system was created by Jiang et al. [21] for the efficient synthesis of 2,4,5-trisubstituted thiazoles explained in Figure 9.
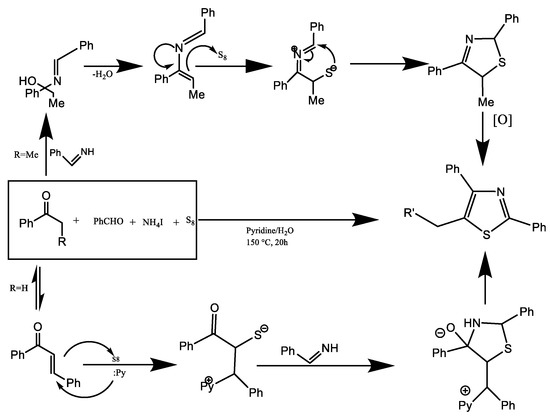
Figure 9.
Synthesis of 2,4,5-trisubstituted thiazoles [21].
2.7. Synthesis of 2-Thio-3-methyl-5-thiazolidinone
After reacting N-methylglycine amide with carbon disulfide in the presence of methanol to produce N-methyl-N-(carbamoylmethyl) ammonium N-methyl-N-(carbamoylmethyl) dithio-carbamate, 2-Thio-3-methyl-5-thiazolidinone derivatives were created. To produce the finished product (Figure 10), this dithiocarbamate was finally acidified using strong HCl or PCl3 [22]. According to reports, a wide range of reactants with the NCS fragment undergo cyclization to produce thiazolidinones when they interact with halocarbonyl compounds. According to reports, a wide range of reactants with the NCS fragment undergo cyclization to produce thiazolidinones when they interact with halocarbonyl compounds.
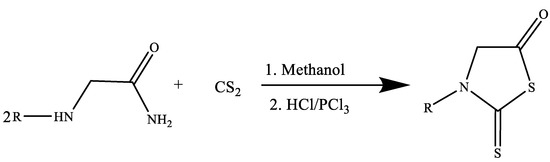
Figure 10.
Synthesis of 2-Thio-3-methyl-5-thiazolidinone derivatives [22].
2.8. Synthesis of 2-Thioxo-4-thiazolidinone
Rhodanine is a member of the important class of chemical compounds known as 2-thioxo-4-thiazolidinone, which has key biological effects. There have been numerous attempts to develop environmentally friendly alternatives to the conventional method of synthesizing rhodanine. One such cutting-edge technology uses a catalyst made of Fe3O4 nanoparticles combined with a biopolymer called carrageenan. Fe3O4@–carrageenan, the resultant nanocomposite, is magnetically active and thus readily separable and reusable. Rhodanine is produced by condensing the reactants amine, CS2, and dialkyl acetylenedicarboxylate in aqueous conditions (Figure 11) [23].
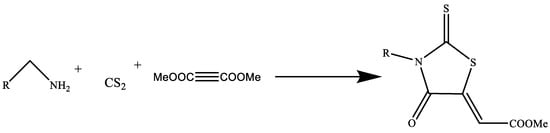
Figure 11.
Green synthesis of rhodamine derivatives [23].
3. Biological Potential of Thiazole
Thiazole derivatives have a broad range of applications in biology and pharmacology and are well-known for their efficacy as pharmaceuticals. They were said to act in an antibacterial [24,25], antioxidant [26], anticancer [25], and antitubercular [25] manner. Thiazole compounds have additionally been investigated as therapeutic candidates for antibacterial agents. The emergence of antibiotic (drug) resistance against bacterial strains has sparked intense interest in the search for and creation of a new and effective antimicrobial medicine. Investigations into thiazole derivatives as an antibacterial agent have been intensively pursued because the thiazole moiety is well-known for its biological action. The introduction of different substituents in the primary molecular framework of thiazole produced encouraging results against the tested bacterial strains [27]. The chemical structures of thiazole derivatives for antimicrobial applications have been extensively studied. The Trichloro phenyl thiazole molecule, in particular, has shown a significant inhibitory impact against a variety of Gram-positive and Gram-negative organisms, including Bacillus subtilis, Escherichia coli, Staphylococcus epidermidis, Staphylococcus aureus, and Pseudomonas fluorescens [28].
A series of 2,4-disubstituted 1,3-thiazole derivatives with distinguishable in vitro antibacterial properties (Figure 12) were designated as a result of the protracted synthesis of active antimicrobial agents [29]. The 36 and 37 analogues that had nitro groups at phenyl substituents showed activity against B. S. subtilis E. coli and A. Compound 38 had MIC values of 4.51, 4.60, and 4.32 compared to E. coli, which had values of 3.92–4.01, 3.39–4.11, and 3.59–4.23, respectively. This is because the studied microorganisms had a nitro moiety at the para position, which forms a powerful hydrogen bond with the amino acid residue. Thus, it may be inferred that the thiazole ring, which has nitro at position 4, was significant in inhibiting the activities of microorganisms and in improving the substituent at the ring [28].
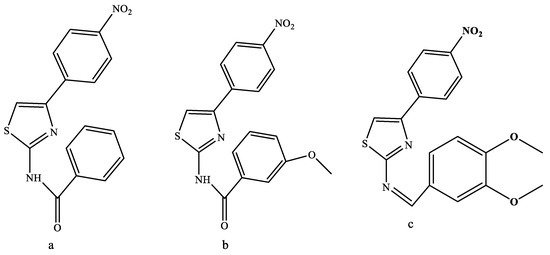
Figure 12.
Series of 2,4-disubstituted thiazole derivatives as antimicrobial agents [28,29].
Another study found that the -C=N spacer in thiazole was beneficial for the compound’s antifungal behavior [30]. The A and B compounds that were synthesized (Figure 13) showed strong antifungal activity against U. P. striiformis and T. tritici.
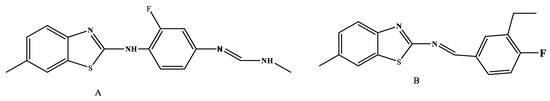
Figure 13.
Series of 2,4-disubstituted thiazole derivatives as antimicrobial agents [30].
4. Conclusions
In conclusion, a wide range of techniques have been reported for the synthesis of thiazole derivatives. The high-yield synthesis of thiazole derivatives can be achieved by developing a novel practical approach. In order to enhance the biological activities, various novel compounds were created and synthesized for use in biological applications by changing the substituent at the thiazole ring with the appropriate substituent groups. Due to its unique characteristics and promise, research on thiazole derivatives may, therefore, be the focus of a further investigation. Therefore, it can be inferred that the thiazole ring with nitro at position 4 played significant roles in inhibiting microorganism activities as well as the optimization of the substituent at the ring [28].
Author Contributions
Conceptualization, S.T. and R.Y.; Synthesis work, S.T., R.Y. and R.S.; writing—review and editing, S.T., R.S., R.Y. and S.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in this article.
Acknowledgments
We would like to convey our obligation to the Faculty and Director of Amity Institute of Pharmacy, Amity University, Amity Education Valley, Gurugram, Manesar, Panchgaon, Haryana 1122412-India, for furnishing all the essential facilities to accomplish the literature review, is gratefully acknowledged.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Huang, K.-C.; Chen, Z.; Jiang, Y.; Akare, S.; Kolber-Simonds, D.; Condon, K.; Agoulnik, S.; Tendyke, K.; Shen, Y.; Wu, K.-M.; et al. Apratoxin A Shows Novel Pancreas-Targeting Activity through the Binding of Sec 61Apratoxin A, a Novel Pancreas-Targeting Agent. Mol. Cancer Ther. 2016, 15, 1208–1216. [Google Scholar] [CrossRef] [PubMed]
- Desai, N.C.; Rajpara, K.M.; Joshi, V.V. Synthesis and antimicrobial screening of 5-(benzylidene)-3-phenylthiazolidin-4-one derivatives incorporating thiazole ring. Med. Chem. Res. 2013, 22, 5044–5055. [Google Scholar] [CrossRef]
- Mahmoodi, N.O.; Pirbasti, F.G. Overview on the recently developed thiazolyl heterocycles as useful therapeutic agents. Phosphorus Sulfur Silicon Relat. Elem. 2016, 191, 811–843. [Google Scholar] [CrossRef]
- Cascioferro, S.M.; Parrino, B.; Carbone, D.; Schillaci, D.; Giovannetti, E.; Cirrincione, G.; Diana, P. Thiazoles, Their Benzofused Systems, and Thiazolidinone Derivatives: Versatile and Promising Tools to Combat Antibiotic Resistance. J. Med. Chem. 2020, 63, 7923–7956. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Chauhan, V.; Silva, J.R.A.; Lameira, J.; D’Andrea, F.B.; Li, S.-G.; Ginell, S.L.; Freundlich, J.S.; Alves, C.N.; Bailey, S.; et al. Mycobacterium abscessus l, d-Transpeptidases Are Susceptible to Inactivation by Carbapenems and Cephalosporins but Not Penicillins. Antimicrob. Agents Chemother. 2017, 61, e00866-17. [Google Scholar] [CrossRef] [PubMed]
- Desai, N.; Joshi, V.; Rajpara, K.; Vaghani, H.; Satodiya, H. Facile synthesis of novel fluorine containing pyrazole based thiazole derivatives and evaluation of antimicrobial activity. J. Fluor. Chem. 2012, 142, 67–78. [Google Scholar] [CrossRef]
- Ripain, I.H.A.; Ngah, N. A brief review on the thiazole derivatives: Synthesis methods and biological activities. Malays. J. Anal. Sci. 2021, 25, 257–267. [Google Scholar]
- Gaballah, S.T.; Khalil, A.M.; Rabie, S.T. Thiazole derivatives-functionalized polyvinyl chloride nanocomposites with photostability and antimicrobial properties. J. Vinyl Addit. Technol. 2019, 25, E137–E146. [Google Scholar] [CrossRef]
- Sharshira, E.M.; Hamada, N.M.M. Synthesis, Characterization and Antimicrobial Activities of Some Thiazole Derivatives. Am. J. Org. Chem. 2012, 2, 69–73. [Google Scholar] [CrossRef]
- Liu, C.-B.; Shan, B.; Bai, H.-M.; Tang, J.; Yan, L.-Z.; Ma, Y.-B. Hydrophilic/hydrophobic characters of antimicrobial peptides derived from animals and their effects on multidrug resistant clinical isolates. Zool. Res. 2015, 36, 41–47. [Google Scholar] [CrossRef]
- Castillo Expósito, J.A. Studies on Antimicrobial Activity of Arginine-Based Surfactants and Chemoenyzmatic Synthesis of Novel Amphiphiles Based on L-Arginine and d-Fagomine; Universitat Autònoma de Barcelona: Bellaterra, Spain, 2007. [Google Scholar]
- Durst, T. Comprehensive Organic Chemistry; Barton, D.H.R., Ollis, W.D., Eds.; Pergamon: Oxford, UK, 1979; Volume 3, p. 197. [Google Scholar]
- Khan, K.M.; Qurban, S.; Salar, U.; Taha, M.; Hussain, S.; Perveen, S.; Hameed, A.; Ismail, N.H.; Riaz, M.; Wadood, A. Synthesis, in vitro α-glucosidase inhibitory activity and molecular docking studies of new thiazole derivatives. Bioorg. Chem. 2016, 68, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Kant, V. A Review on Biological Activity of Imidazole and Thiazole Moieties and their Derivatives. Sci. Int. 2013, 1, 253–260. [Google Scholar] [CrossRef]
- Mishra, C.B.; Kumari, S.; Tiwari, M. Thiazole: A promising heterocycle for the development of potent CNS active agents. Eur. J. Med. Chem. 2015, 92, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Sattar, N.E.A.; El-Naggar, A.M.; Abdel-Mottaleb, M.S.A. Novel Thiazole Derivatives of Medicinal Potential: Synthesis and Modeling. J. Chem. 2017, 2017, 4102796. [Google Scholar] [CrossRef]
- Kumar, J.S.; Alam, M.A.; Gurrapu, S.; Nelson, G.; Williams, M.; Corsello, M.A.; Johnson, J.L.; Jonnalagadda, S.C.; Mereddy, V.R. Synthesis and Biological Evaluation of Novel Benzoxaboroles as Potential Antimicrobial and Anticancer Agents. J. Heterocycl. Chem. 2013, 50, 814–820. [Google Scholar] [CrossRef]
- de Santana, T.I.; Barbosa, M.D.O.; Gomes, P.A.T.D.M.; da Cruz, A.C.N.; da Silva, T.G.; Leite, A.C.L. Synthesis, anticancer activity and mechanism of action of new thiazole derivatives. Eur. J. Med. Chem. 2018, 144, 874–886. [Google Scholar] [CrossRef]
- Wu, M.; Jiang, Y.; An, Z.; Qi, Z.; Yan, R. Iron-Catalyzed Synthesis of Substituted Thiazoles from Enamines and Elemental Sulfur through C−S Bond Formation. Adv. Synth. Catal. 2018, 360, 4236–4240. [Google Scholar] [CrossRef]
- Gomha, S.M.; Salaheldin, T.A.; Hassaneen, H.M.E.; Abdel-Aziz, H.M.; Khedr, M.A. Synthesis, Characterization and Molecular Docking of Novel Bioactive Thiazolyl-Thiazole Derivatives as Promising Cytotoxic Antitumor Drug. Molecules 2015, 21, 3. [Google Scholar] [CrossRef]
- Jiang, J.; Huang, H.; Deng, G.J. Four-component thiazole formation from simple chemicals under metal-free conditions. Green Chem. 2019, 21, 986–990. [Google Scholar] [CrossRef]
- Cook, A.H.; Cox, S.F. Studies in the azole series. Part XXI. Experiments with N-alkylamino-nitriles. J. Chem. Soc. (Resumed) 1949, 495, 2337–2341. [Google Scholar] [CrossRef]
- Rostamnia, S.; Zeynizadeh, B.; Doustkhah, E.; Baghban, A.; Aghbash, K.O. The use of κ-carrageenan/Fe3O4 nanocomposite as a nanomagnetic catalyst for clean synthesis of rhodanines. Catal. Commun. 2015, 68, 77–83. [Google Scholar] [CrossRef]
- Darwish, E.S.; Fattah, A.M.A.; Attaby, F.A.; Al-Shayea, O.N. Synthesis and Antimicrobial Evaluation of Some Novel Thiazole, Pyridone, Pyrazole, Chromene, Hydrazone Derivatives Bearing a Biologically Active Sulfonamide Moiety. Int. J. Mol. Sci. 2014, 15, 1237–1254. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, M.E. Recent Developments and Biological Activities of 2-Aminothiazole Derivatives. Acta Chim. Slov. 2018, 65, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Jha, A.K.; Shukla, P.K.; Soni, N.; Verma, A. Synthesis, characterization and biological evaluation of thiazole in-corporated triazole compounds. Pharm. Lett. 2015, 7, 67–74. [Google Scholar]
- Catalano, A.; Carocci, A.; Defrenza, I.; Muraglia, M.; Carrieri, A.; Van Bambeke, F.; Rosato, A.; Corbo, F.; Franchini, C. 2-Aminobenzothiazole derivatives: Search for new antifungal agents. Eur. J. Med. Chem. 2013, 64, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Ayati, A.; Emami, S.; Asadipour, A.; Shafiee, A.; Foroumadi, A. Recent applications of 1,3-thiazole core structure in the identification of new lead compounds and drug discovery. Eur. J. Med. Chem. 2015, 97, 699–718. [Google Scholar] [CrossRef]
- Arora, P.; Narang, R.; Bhatia, S.; Nayak, S.; Singh, S.; Narasimhan, B. Synthesis, molecular docking and QSAR studies of 2, 4-disubstituted thiazoles as antimicrobial agents. J. Appl. Pharm. Sci. 2015, 5, 28–42. [Google Scholar] [CrossRef]
- Sidhu, A.; Kukreja, S. Synthesis of novel fluorinated benzothiazol-2-yl-1,2,4-triazoles: Molecular docking, antifungal evaluation and in silico evaluation for SAR. Arab. J. Chem. 2019, 12, 2118–2127. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).