Semisynthesis of 6β-Acetoxyvouacapane Derivatives via the Ugi-Azide Multicomponent Reaction †
Abstract
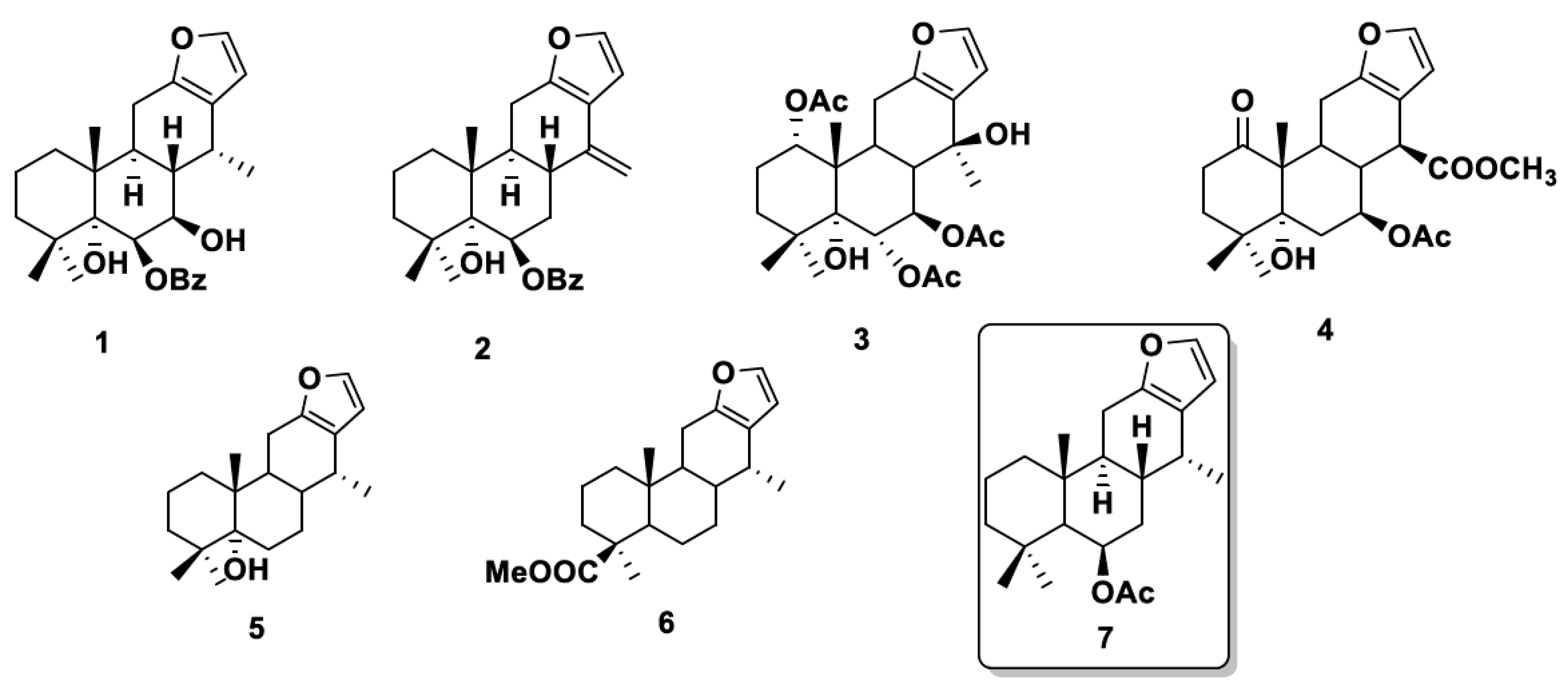
1. Introduction
2. Materials and Methods
2.1. Experimental Section
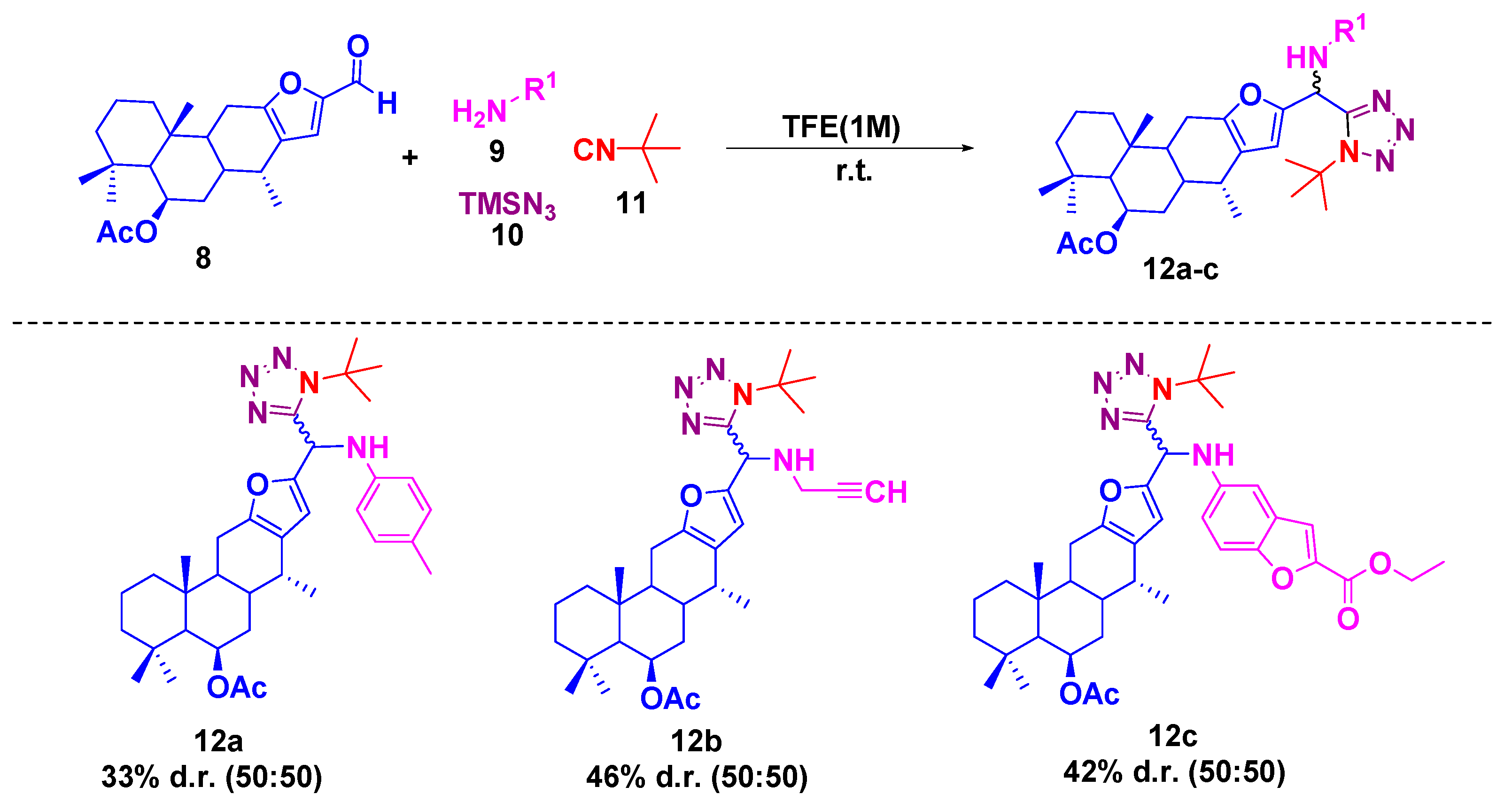
2.2. General Procedure for 1,5-Disubstituted Tetrazoles-Vouacapane 12a–c
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Davisona, E.K.; Brimblea, M.A. Natural product derived privileged scaffolds in drug discovery. Curr. Opin. Chem. Biol. 2019, 52, 1. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F.; Vian, M.A.; Cravotto, G. Green Extraction of Natural Products: Concept and Principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef] [PubMed]
- Koparde, A.; Doijad, R.; Magdum, C. Natural Products in Drug Discovery. In Pharmacognosy-Medicinal Plants; Shagufta, P., Areej, A.-T., Eds.; IntechOpen: London, UK, 2019. [Google Scholar]
- Song, L.; Cai, L.; Van der Eycken, E.V. Mic Microwave-Assisted Post-Ugi Reactions for the Synthesis of Polycycles. Molecules 2022, 27, 1. [Google Scholar]
- Fouad, M.A.; Abdel-Hamid, H.; Ayoup, M.S. Two decades of recent advances of Ugi reactions: Synthetic and pharmaceutical applications. RSC Adv. 2020, 10, 42644. [Google Scholar] [CrossRef] [PubMed]
- Ibarra, I.; Islas-Jácome, A.; González-Zamora, E. Synthesis of polyheterocycles via multicomponent reactions. Org. Biomol. Chem. 2017, 16, 1402. [Google Scholar] [CrossRef] [PubMed]
- Gagnon, E.; Bruneau, A.; Hughes, C.E.; de Queiroz, L.P.; Lewis, G.P. A new generic system for the pantropical Caesalpinia group (Leguminosae). PhytoKeys 2016, 71, 1. [Google Scholar] [CrossRef] [PubMed]
- Jing, W.; Zhang, X.; Zhou, H.; Wang, Y.; Yang, M.; Long, L.; Gao, H. Naturally occurring cassane diterpenoids (CAs) of Caesalpinia: A systematic review of its biosynthesis, chemistry and pharmacology. Fitoterapia 2019, 134, 226. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Hurtado, M.A.; Álvarez-Esquivel, F.E.; Rodríguez-García, G.; Martínez-Pacheco, M.M.; Espinoza-Madrigal, R.M.; Pamatz-Bolaños, T.; Salvador-Hernández, J.L.; García-Gutiérrez, H.A.; Cerda-García-Rojas, C.M.; Joseph-Nathan, P.; et al. Cassane diterpenes from Caesalpinia platyloba. Phytochemistry 2013, 96, 397. [Google Scholar] [CrossRef] [PubMed]
- Niño-Pantoja, I.; Gallardo-Alfonzo, A.; Solis-Santos, M.; Ordoñez, M.; Contreras-Celedón, C.; Islas-Jácome, A.; Chacón-García, L.; Cortés-García, C.J. Synthesis of 1,5-Disubstituted Tetrazole−Indolizine Bis-Heterocycles and Their Copper (II) Recognizing Properties. Eur. J. Org. Chem. 2022, 34, e202200230. [Google Scholar] [CrossRef]
- Aguilar-Morales, C.M.; Araujo-Huitrado, J.G.; López-Hernández, Y.; Contreras-Celedón, C.; Islas-Jácome, A.; Granados-López, A.J.; Solorio-Alvarado, C.R.; Adrián-López, J.; Chacón-García, L.; Cortés-García, J.C. A One-Pot Six-Component Reaction for the Synthesis of 1,5-Disubstituted Tetrazol-1,2,3-Triazole Hybrids and Their Cytotoxic Activity against the MCF-7 Cell Line. Molecules 2021, 26, 6104. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Morales, C.M.; de Loera, D.; Contreras-Celedón, C.; Cortés-García, C.J.; Chacón-García, L. Synthesis of 1,5-disubstituted Tetrazole-1,2,3 Triazoles Hybrids Via Ugi-azide/CuAAC. Synthetic Commun. 2019, 49, 2086. [Google Scholar] [CrossRef]
- Cortes-García, C.J.; Islas-Jácome, A.; Rentería-Gómez, A.; Montaño-Gámez, R. Synthesis of 1,5-disubstituted Tetrazoles Containing a Fragment of the Anticancer Drug Imatinib Via a Microwave-Assisted Ugi-azide Reaction. Monatsh Chem. 2016, 147, 1277. [Google Scholar] [CrossRef]
- Unnamatla, M.V.B.; Islas-Jácome, A.; Quezada-Soto, A.; Ramírez-López, S.C.; Flores-Alamo, M.; Gámez-Montaño. Multicomponent One-Pot Synthesis of 3-Tetrazolyl and 3-Imidazo [1,2-a]pyridin Tetrazolo [1,5-a]quinolines. J. Org. Chem. 2016, 81, 10576. [Google Scholar] [CrossRef] [PubMed]
- Barreto, A.; Dos Santos, V.; Andrade, C. Consecutive Hydrazino-Ugi-azide Reactions: Synthesis of Acylhydrazines Bearing 1,5-disubstituted Tetrazoles. Beilstein J. Org. Chem. 2017, 13, 2596. [Google Scholar] [CrossRef] [PubMed]
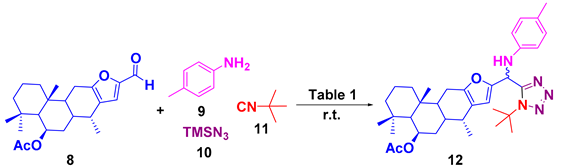 | ||
|---|---|---|
| Entry a | Solvent | Yield (%) |
| 1 | MeOH (1M) | ND b |
| 2 | 2,2,2-trifluroethanol (1M) | 33 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Servín-García, G.; Chacón-García, L.; González-Marrero, J.; Macías-Alonso, M.; Gómez-Hurtado, M.A.; Rodríguez-García, G.; del Río, R.E.; Cortés-García, C.J. Semisynthesis of 6β-Acetoxyvouacapane Derivatives via the Ugi-Azide Multicomponent Reaction. Chem. Proc. 2022, 12, 24. https://doi.org/10.3390/ecsoc-26-13552
Servín-García G, Chacón-García L, González-Marrero J, Macías-Alonso M, Gómez-Hurtado MA, Rodríguez-García G, del Río RE, Cortés-García CJ. Semisynthesis of 6β-Acetoxyvouacapane Derivatives via the Ugi-Azide Multicomponent Reaction. Chemistry Proceedings. 2022; 12(1):24. https://doi.org/10.3390/ecsoc-26-13552
Chicago/Turabian StyleServín-García, Gabriela, Luis Chacón-García, Joaquín González-Marrero, Mariana Macías-Alonso, Mario A. Gómez-Hurtado, Gabriela Rodríguez-García, Rosa E. del Río, and Carlos J. Cortés-García. 2022. "Semisynthesis of 6β-Acetoxyvouacapane Derivatives via the Ugi-Azide Multicomponent Reaction" Chemistry Proceedings 12, no. 1: 24. https://doi.org/10.3390/ecsoc-26-13552
APA StyleServín-García, G., Chacón-García, L., González-Marrero, J., Macías-Alonso, M., Gómez-Hurtado, M. A., Rodríguez-García, G., del Río, R. E., & Cortés-García, C. J. (2022). Semisynthesis of 6β-Acetoxyvouacapane Derivatives via the Ugi-Azide Multicomponent Reaction. Chemistry Proceedings, 12(1), 24. https://doi.org/10.3390/ecsoc-26-13552






