Synthesis of Bis (1,4-Disubstituted-1,2,3-triazoles) Starting from Diethyl Galactarate †
Abstract
:1. Introduction
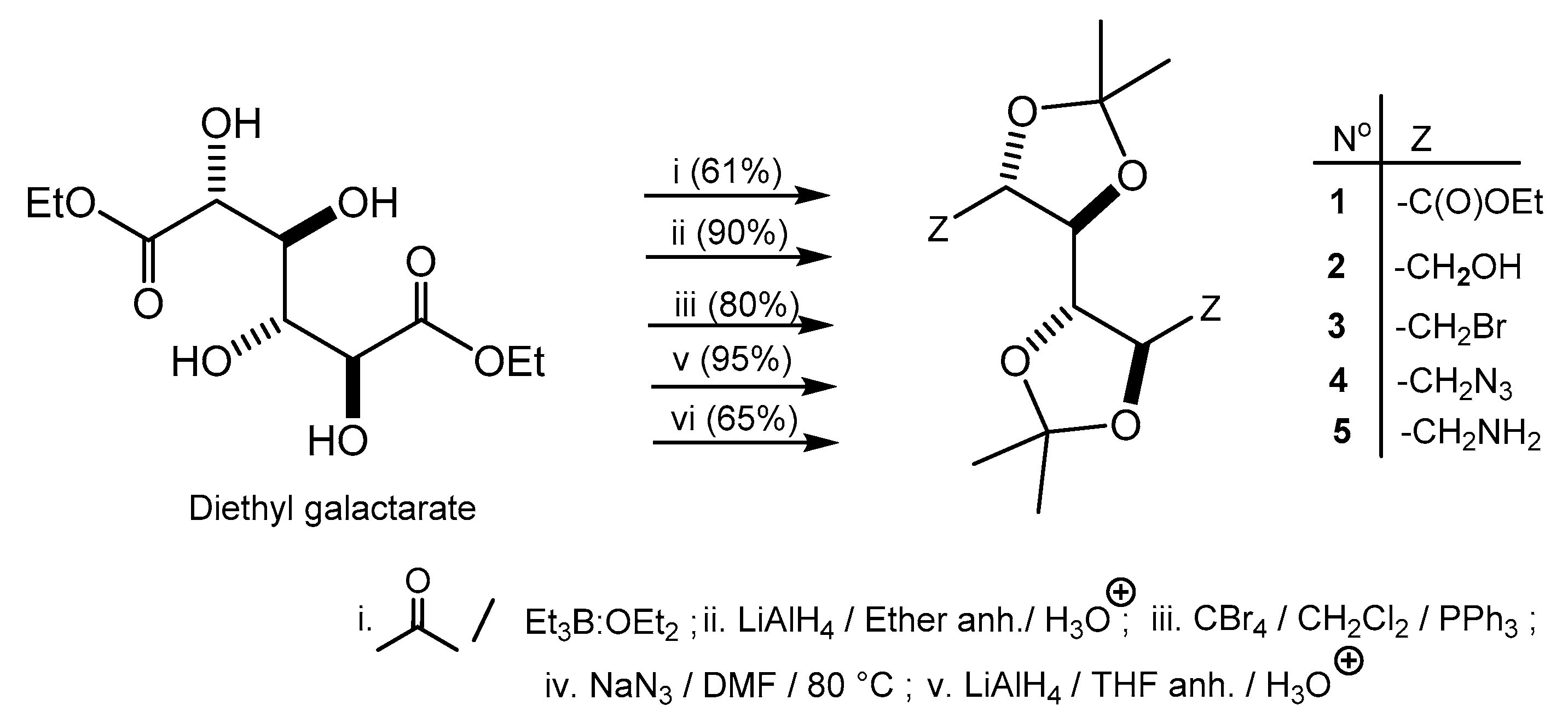
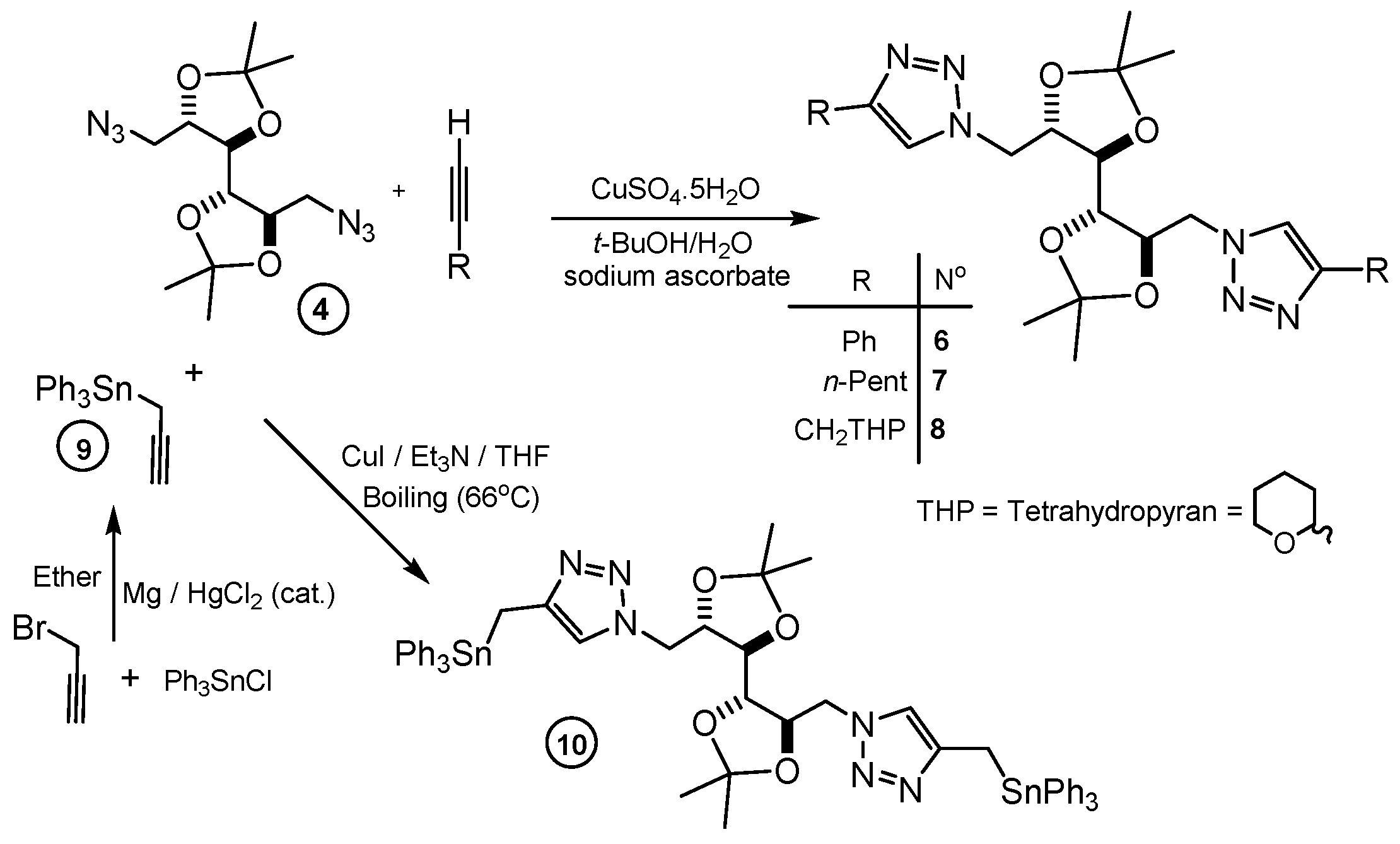
2. Results and Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khan, M.F.; Verma, G.; Akhtar, W.; Marella, A.; Alam, M.M.; Mumtaz, A.M.; Husain, A.; Hasan, S.M.; Shaquiquzzaman, M.H.; Syed, R. Synthetic Trends Followed for the Development of 1,2,3-Triazole Derivatives. Int. J. Drug. Dev. Res. 2017, 9, 22–25. [Google Scholar]
- Zacconi, F.C.; Romina, A.; Ocampo, R.A.; Podestá, J.C.; Koll, L.C. Synthesis of organotin substituted tricyclic macrodiolides. J. Braz. Chem. Soc. 2016, 27, 484–492. [Google Scholar] [CrossRef]
- Scoccia, J.; Gerbino, D.C.; Podestá, J.C. Synthesis of new organotin substituted optically active eleven membered macrodiolides. Tetrahedron Asym. 2016, 27, 352–360. [Google Scholar] [CrossRef]
- Terraza, V.F.; Gerbino, D.C.; Podestá, J.C. Synthesis of new mixed (-)-menthylalkyltin dihydrides. Stereoselective reduction of chiral and prochiral ketones. J. Organomet. Chem. 2021, 935, 121680. [Google Scholar] [CrossRef]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Huisgen, R. Kinetics and reaction mechanisms: Selected examples from the experience of forty years. Pure Appl. Chem. 1989, 61, 613–628. [Google Scholar] [CrossRef]
- Prömpers, G.; Keul, H.; Höcker, H. Polyurethanes with pendant hydroxy groups: Polycondensation of 1,6-bis-O-phenoxycarbonyl-2,3:4,5-di-O-isopropylidenegalactitol and 1,6-di-O-phenoxycarbonylgalactitol with diamines. Green Chem. 2006, 8, 467. [Google Scholar] [CrossRef]
- Rostovtsev, V.V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A Stepwise Huisgen Cycloaddition Process: Copper(I)-Catalyzed Regioselective “Ligation” of Azides and Terminal Alkynes. Angew. Chem. Int. Ed. 2002, 41, 2423–2618. [Google Scholar] [CrossRef]
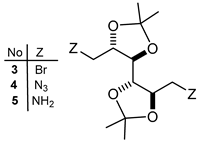
| Comp. N° | Chemical Shifts (δ, ppm) a |
|---|---|
| 3 | 1.41 (s, 6H); 1.77 (s, 6H); 3.52 (m, 2H); 3.69 (m, 2H); 3.82 (m, 2H) |
| 4 | 1.31 (s, 6H); 1.38 (s, 6H); 3.22–3.28 (m, 2H); 3.56–3.62 (m, 2H); 3.70–3.73 (m, 2H); 4.03–4.09 (m, 2H) |
| 5 | 1.29 (s, 6H); 1.32 (s, 6H); 2.15 (s, 4H; NH2 × 2); 2.76 (m, 2H); 2.90 (m, 2H); 3.57 (m, 2H); 3.90 (m, 2H) |
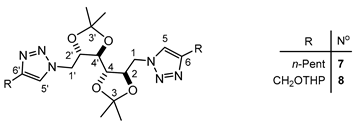
| Comp. N° | Me | C-1 y 1′ | C-2 y 2′ | C-3 y 3′ | C-4 y 4′ | C-5 y 5′ | C-6 y 6′ |
|---|---|---|---|---|---|---|---|
| 7 b | 26.99 | 51.10 | 79.09 | 111.01 | 78.27 | 122.42 | 148.45 |
| 8 c | 26.93 | 51.04 | 78.93 | 111.00 | 78.14 | 121.19 | 149.53 |
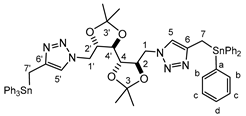
| Comp. N° 10 | Me | C(1/1′) | C(2/2′) | C(3/3′) | C(4/4′) | C(5/5′) | C(6/6′) | C(7/7′) |
|---|---|---|---|---|---|---|---|---|
| 13C-NMR b | 26.86 | 51.11 | 78.09 | 110.80 | 79.06 | 121.55 (26.8) | 145.98 (33.9) | 7.79 (349.0) |
| 1H-NMR | 1.09 (s, 6H); 1.19 (s, 6H); 2.83 [s, 4H, 2J(Sn,H) = 60.9 Hz]; 3.28–3.49 (m, 2H); 3.89–4.11 (m, 3H); 4.15–4.34 (m, 2H); 4.40–4.60 (m, 2H); 7.00–7.60 (m c, 32H) | |||||||
| 119Sn-NMR | 113.66 | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terraza, V.; Gerbino, D.; Podestá, J. Synthesis of Bis (1,4-Disubstituted-1,2,3-triazoles) Starting from Diethyl Galactarate. Chem. Proc. 2022, 12, 22. https://doi.org/10.3390/ecsoc-26-13689
Terraza V, Gerbino D, Podestá J. Synthesis of Bis (1,4-Disubstituted-1,2,3-triazoles) Starting from Diethyl Galactarate. Chemistry Proceedings. 2022; 12(1):22. https://doi.org/10.3390/ecsoc-26-13689
Chicago/Turabian StyleTerraza, Víctor, Darío Gerbino, and Julio Podestá. 2022. "Synthesis of Bis (1,4-Disubstituted-1,2,3-triazoles) Starting from Diethyl Galactarate" Chemistry Proceedings 12, no. 1: 22. https://doi.org/10.3390/ecsoc-26-13689
APA StyleTerraza, V., Gerbino, D., & Podestá, J. (2022). Synthesis of Bis (1,4-Disubstituted-1,2,3-triazoles) Starting from Diethyl Galactarate. Chemistry Proceedings, 12(1), 22. https://doi.org/10.3390/ecsoc-26-13689






