Beneficial Role of Increased Glucose Infusion in Decompensated Type 2 Diabetes Patient
Abstract
1. Introduction
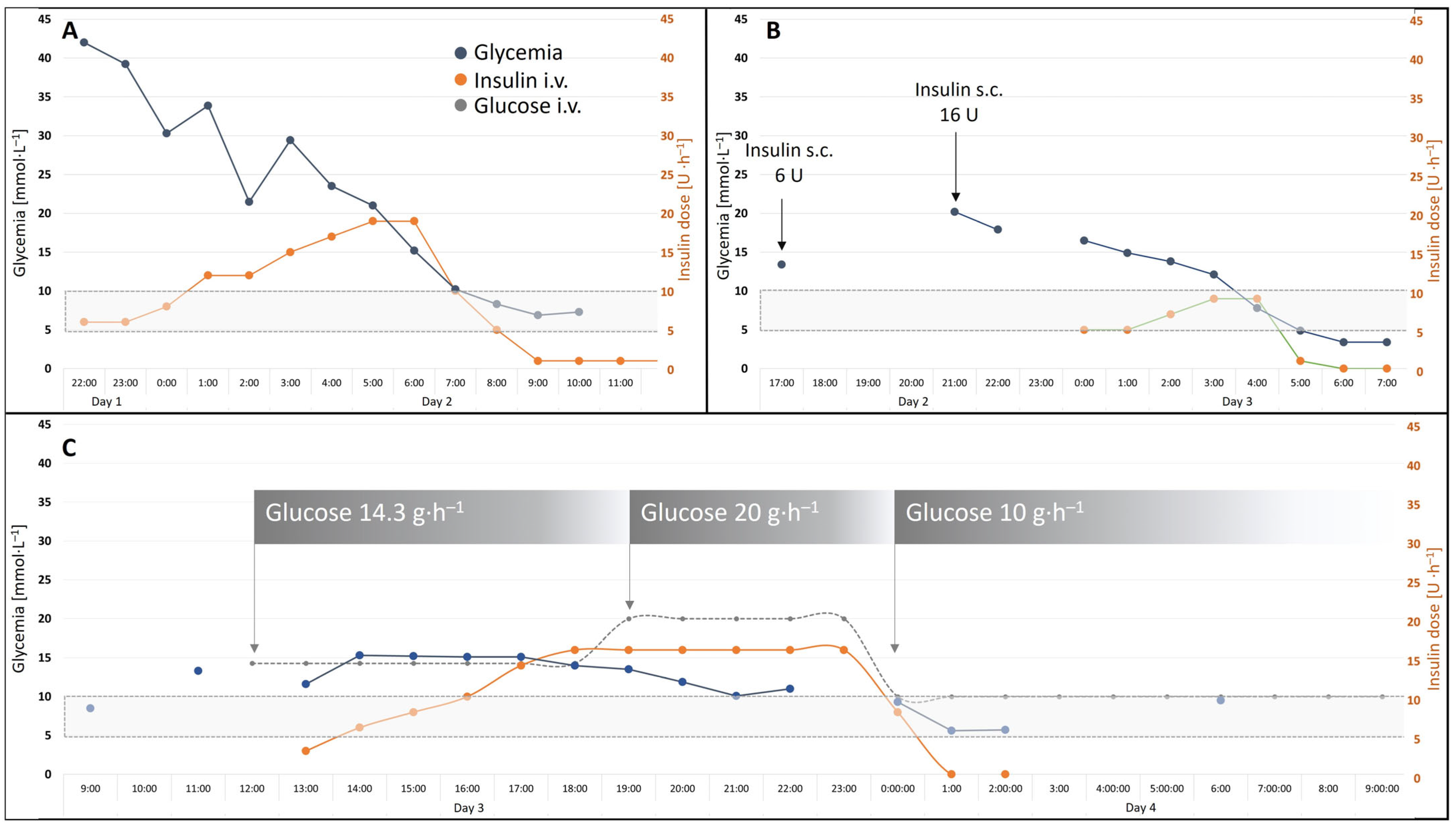
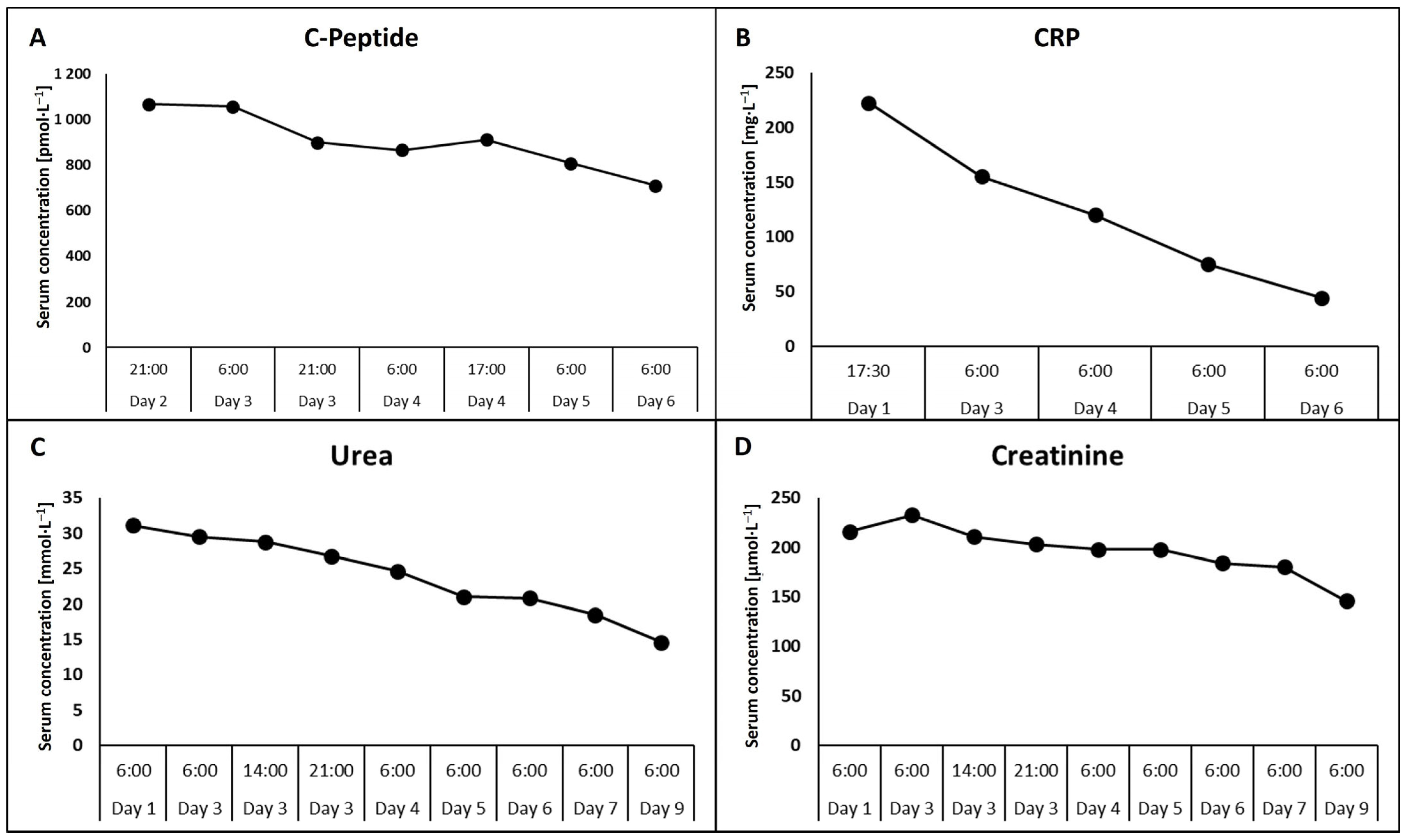
2. Case Report
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CHO | Carbohydrate |
| GIK | Glucose–insulin–potassium |
| Glc | Glucose |
| CRP | C-reactive protein |
| ICU | Intensive care unit |
| IL-6 | Interleukin 6 |
| IV | Intravenous |
| IR | Insulin resistance |
| PO | Per oral |
| SC | Subcutaneous |
| T2DM | Type 2 diabetes mellitus |
| TNF- α | Tumor necrosis factor alpha |
References
- Soeters, P.B.; Shenkin, A.; Sobotka, L.; Soeters, M.R.; de Leeuw, P.W.; Wolfe, R.R. The anabolic role of the Warburg, Cori-cycle and Crabtree effects in health and disease. Clin. Nutr. 2021, 40, 2988–2998. [Google Scholar] [CrossRef] [PubMed]
- Sobotka, O.; Ticha, M.; Kubickova, M.; Adamek, P.; Polakova, L.; Mezera, V.; Sobotka, L. Should Carbohydrate Intake Be More Liberal during Oral and Enteral Nutrition in Type 2 Diabetic Patients? Nutrients 2023, 15, 439. [Google Scholar] [CrossRef]
- Sobotka, L.; Sobotka, O. The predominant role of glucose as a building block and precursor of reducing equivalents. Curr. Opin. Clin. Nutr. Metab. Care 2021, 24, 555–562. [Google Scholar] [CrossRef]
- Petersen, M.C.; Shulman, G.I. Mechanisms of Insulin Action and Insulin Resistance. Physiol. Rev. 2018, 98, 2133–2223. [Google Scholar] [CrossRef]
- Soeters, M.R.; Soeters, P.B. The evolutionary benefit of insulin resistance. Clin. Nutr. 2012, 31, 1002–1007. [Google Scholar] [CrossRef]
- Xiao, W.; Wang, R.S.; Handy, D.E.; Loscalzo, J. NAD(H) and NADP(H) Redox Couples and Cellular Energy Metabolism. Antioxid. Redox Signal. 2018, 28, 251–272. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.S.; Cha, Y.H.; Kim, H.S.; Kim, N.H.; Yook, J.I. The Pentose Phosphate Pathway as a Potential Target for Cancer Therapy. Biomol. Ther. 2018, 26, 29–38. [Google Scholar] [CrossRef]
- Hammad, N.; Rosas-Lemus, M.; Uribe-Carvajal, S.; Rigoulet, M.; Devin, A. The Crabtree and Warburg effects: Do metabolite-induced regulations participate in their induction? Biochim. Biophys. Acta 2016, 1857, 1139–1146. [Google Scholar] [CrossRef] [PubMed]
- Cori, C.F. The glucose-lactic acid cycle and gluconeogenesis. Curr. Top. Cell Regul. 1981, 18, 377–387. [Google Scholar]
- Wang, Y.; Lin, S.; Chuang, Y.; Sheu, W.; Tung, K.; Chen, C. Activation of hepatic inflammatory pathways by catecholamines is associated with hepatic insulin resistance in male ischemic stroke rats. Endocrinology 2014, 155, 1235–1246. [Google Scholar] [CrossRef]
- Rizza, R.; Mandarino, L.; Gerich, J. Cortisol-induced insulin resistance in man: Impaired suppression of glucose production and stimulation of glucose utilization due to a postreceptor defect of insulin action. J. Clin. Endocrinol. Metab. 1982, 54, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Kirsch, D.; Baumgarten, M.; Deufel, T.; Rinninger, F.; Kemmler, W.; Haring, H. Catecholamine-induced insulin resistance of glucose transport in isolated rat adipocytes. Biochem. J. 1983, 216, 737–745. [Google Scholar] [CrossRef]
- Hotamisligil, G.S.; Murray, D.L.; Choy, L.N.; Spiegelman, B.M. Tumor necrosis factor alpha inhibits signaling from the insulin receptor. Proc. Natl. Acad. Sci. USA 1994, 91, 4854–4858. [Google Scholar] [CrossRef]
- Skorepa, P.; Sobotka, O.; Vanek, J.; Ticha, A.; Fortunato, J.; Manak, J.; Blaha, V.; Horacek, J.M.; Sobotka, L. The Impact of Glucose-Based or Lipid-Based Total Parenteral Nutrition on the Free Fatty Acids Profile in Critically Ill Patients. Nutrients 2020, 12, 1373. [Google Scholar] [CrossRef] [PubMed]
- Kotfis, K.; Jamioł-Milc, D.; Skonieczna-Żydecka, K.; Folwarski, M.; Stachowska, E. The effect of preoperative carbohydrate loading on clinical and biochemical outcomes after cardiac surgery: A systematic review and meta-analysis of randomized trials. Nutrients 2020, 12, 3105. [Google Scholar] [CrossRef]
- Świderska, E.; Strycharz, J.; Wróblewski, A.; Szemraj, J.; Drzewoski, J.; Śliwińska, A. Role of PI3K/AKT Pathway in Insulin-Mediated Glucose Uptake. In Blood Glucose Levels, 1st ed.; Leszek, S., Ed.; Intech Open: Rijeka, Croatia, 2018; Chapter 3. [Google Scholar]
- Garcia, S.M.; Hirschberg, P.R.; Sarkar, P.; Siegel, D.M.; Teegala, S.B.; Vail, G.M.; Routh, V.H. Insulin actions on hypothalamic glucose-sensing neurones. J. Neuroendocrinol. 2021, 33, e12937. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.Y.; Lawrence-Sidebottom, D.; Piotrowska, K.; Zhang, W.; Iranmanesh, A.; Auchus, R.J.; Veldhuis, J.D.; Van Dongen, H.P.A. Clamping Cortisol and Testosterone Mitigates the Development of Insulin Resistance during Sleep Restriction in Men. J. Clin. Endocrinol. Metab. 2021, 106, e3436–e3448. [Google Scholar] [CrossRef]
- Buchanan, T.A.; Xiang, A.H.; Peters, R.K.; Kjos, S.L.; Berkowitz, K.; Marroquin, A.; Goico, J.; Ochoa, C.; Azen, S.P. Response of pancreatic beta-cells to improved insulin sensitivity in women at high risk for type 2 diabetes. Diabetes 2000, 49, 782–788. [Google Scholar] [CrossRef]
- Mehran, L.; Amouzegar, A.; Azizi, F. Thyroid disease and the metabolic syndrome. Curr. Opin. Endocrinol. Diabetes Obes. 2019, 26, 256–265. [Google Scholar] [CrossRef]
- D’Elia, J.; Mulla, C.; Liu, J.; Weinrauch, L. Variations in glucose/C-peptide ratio in patients with type 2 diabetes associated with renal function. Diabetes Res. Clin. Pract. 2019, 150, 1–7. [Google Scholar] [CrossRef]
- Kwon, S.; Park, J.; Park, S.; Lee, Y.; Kim, G.; Hur, K.; Kim, J.; Jin, S. Plasma C-peptide levels and the continuous glucose monitoring-defined coefficient of variation in risk prediction for hypoglycemia in Korean people with diabetes having normal and impaired kidney function. Endocrinol. Metab. 2025, 40, 268. [Google Scholar] [CrossRef] [PubMed]
- Qaseem, A.; Humphrey, L.L.; Chou, R.; Snow, V.; Shekelle, P.; Clinical Guidelines Committee of the American College of Physicians. Use of intensive insulin therapy for the management of glycemic control in hospitalized patients: A clinical practice guideline from the American College of Physicians. Ann. Intern. Med. 2011, 154, 260–267. [Google Scholar] [CrossRef]
- Lipscombe, L.; Booth, G.; Butalia, S.; Dasgupta, K.; Eurich, D.T.; Goldenberg, R.; Khan, N.; MacCallum, L.; Shah, B.R.; Simpson, S. Pharmacologic glycemic management of type 2 diabetes in adults. Can. J. Diabetes 2018, 42 (Suppl. S1), S88–S103. [Google Scholar] [CrossRef] [PubMed]
- Diabetes Canada Clinical Practice Guidelines Expert Committee; Lipscombe, L.; Butalia, S.; Dasgupta, K.; Eurich, D.T.; MacCallum, L.; Shah, B.R.; Simpson, S.; Senior, P.A. Pharmacologic glycemic management of type 2 diabetes in adults: 2020 update. Can. J. Diabetes 2020, 44, 575–591. [Google Scholar] [CrossRef]
- Scott, M.G.; Bruns, D.E.; Boyd, J.C.; Sacks, D.B. Tight glucose control in the intensive care unit: Are glucose meters up to the task? Clin. Chem. 2009, 55, 18–20. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. 16. Diabetes care in the hospital: Standards of care in diabetes—2025. Diabetes Care 2025, 48 (Suppl. S1), S321–S334. [Google Scholar] [CrossRef]
- Singer, P.; Blaser, A.R.; Berger, M.M.; Calder, P.C.; Casaer, M.; Hiesmayr, M.; Mayer, K.; Montejo-Gonzalez, J.C.; Pichard, C.; Preiser, J.C.; et al. ESPEN practical and partially revised guideline: Clinical nutrition in the intensive care unit. Clin. Nutr. 2023, 42, 1671–1689. [Google Scholar] [CrossRef]
- Honarmand, K.; Sirimaturos, M.; Hirshberg, E.L.; Bircher, N.G.; Agus, M.S.D.; Carpenter, D.L.; Downs, C.R.; Farrington, E.A.; Freire, A.X.; Grow, A.; et al. Society of Critical Care Medicine guidelines on glycemic control for critically ill children and adults 2024. Crit. Care Med. 2024, 52, e161–e181. [Google Scholar] [CrossRef] [PubMed]
- NICE-SUGAR Study Investigators; Finfer, S.; Liu, B.; Chittock, D.R.; Norton, R.; Myburgh, J.A.; McArthur, C.; Mitchell, I.; Foster, D.; Dhingra, V.; et al. Hypoglycemia and risk of death in critically ill patients. N. Engl. J. Med. 2012, 367, 1108–1118. [Google Scholar] [CrossRef]
- Gunst, J.; Debaveye, Y.; Güiza, F.; Dubois, J.; De Bruyn, A.; Dauwe, D.; De Troy, E.; Casaer, M.; De Vlieger, G.; Haghedooren, R.; et al. Tight blood-glucose control without early parenteral nutrition in the ICU. N. Engl. J. Med. 2023, 389, 1180–1190. [Google Scholar] [CrossRef]
- Defante, M.; Mendes, B.; De Souza, M.; De Hollanda Morais, B.; Martins, O.; Prizão, V.; Parolin, S. Tight versus liberal blood glucose control in patients with diabetes in the ICU: A meta-analysis of randomized controlled trials. J. Intensive Care Med. 2024. [Google Scholar] [CrossRef] [PubMed]
- Krinsley, J.; Chase, J.; Gunst, J.; Mårtensson, J.; Schultz, M.; Taccone, F.; Wernerman, J.; Bohé, J.; Block, C.; Desaive, T.; et al. Continuous glucose monitoring in the ICU: Clinical considerations and consensus. Crit. Care 2017, 21, 197. [Google Scholar] [CrossRef] [PubMed]
- Bronsveld, W.; Van Den Bos, G.; Thijs, L. Use of glucose–insulin–potassium (GIK) in human septic shock. Crit. Care Med. 1985, 13, 566–570. [Google Scholar] [CrossRef] [PubMed]
- Effat, H.; Khaled, R.; Battah, A.; Shehata, M.; Farouk, W. Effect of glucose–insulin–potassium infusion on hemodynamics in patients with septic shock. Open Access Maced. J. Med. Sci. 2021, 9, 1517–1524. [Google Scholar] [CrossRef]
- Kim, W.; Baek, M.; Kim, Y.; Seo, J.; Huh, J.; Lim, C.; Koh, Y.; Hong, S. Glucose–insulin–potassium correlates with hemodynamic improvement in patients with septic myocardial dysfunction. J. Thorac. Dis. 2016, 8, 3648–3657. [Google Scholar] [CrossRef]
- Horst, I.; Ligtenberg, J.J.M.; Bilo, H.J.G.; Zijlstra, F.; Gans, R.O.B. Glucose–insulin–potassium infusion in sepsis and septic shock: No hard evidence yet. Crit. Care 2003, 7, 13–15. [Google Scholar] [CrossRef]
- Schorer, R.; Putzu, A.; Keli-Barcelos, G.; Licker, M. Glucose–insulin–potassium (GIK) morbidity and mortality effects in cardiac surgery: A systematic review and meta-analysis of randomized trials. J. Cardiothorac. Vasc. Anesth. 2020, 34, S40–S41. [Google Scholar] [CrossRef]
- Hamdulay, S.S.; Khafaji, A.A.; Montgomery, H. Glucose–insulin and potassium infusions in septic shock. Chest 2006, 129, 800–804. [Google Scholar] [CrossRef]
- Zhao, K.; Zhang, Y.; Li, J.; Cui, Q.; Zhao, R.; Chen, W.; Liu, J.; Zhao, B.; Wan, Y.; Yu, S.; et al. Modified glucose–insulin–potassium regimen provides cardioprotection with improved tissue perfusion in patients undergoing cardiopulmonary bypass surgery. J. Am. Heart Assoc. 2020, 9, e012376. [Google Scholar] [CrossRef]
- Diaz, R.; Piegas, L.; Tajer, C.; Moreno, M.; Corvalán, R.; Isea, J.; Romero, G. Metabolic modulation of acute myocardial infarction: The ECLA glucose–insulin–potassium pilot trial. Circulation 1998, 98, 2227–2234. [Google Scholar] [CrossRef]
- Rusavy, Z.; Macdonald, I.A.; Sramek, V.; Lacigova, S.; Tesinsky, P.; Novak, I. Glycemia influences on glucose metabolism in sepsis during hyperinsulinemic clamp. JPEN J. Parenter. Enteral Nutr. 2005, 29, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Rusavy, Z.; Sramek, V.; Lacigova, S.; Novak, I.; Tesinsky, P.; Macdonald, I.A. Influence of insulin on glucose metabolism and energy expenditure in septic patients. Crit. Care 2004, 8, R213–R220. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, R.R.; Martini, W.Z. Changes in intermediary metabolism in severe surgical illness. World J. Surg. 2000, 24, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Noba, L.; Wakefield, A. Are carbohydrate drinks more effective than preoperative fasting: A systematic review of randomised controlled trials. J. Clin. Nurs. 2019, 28, 3096–3116. [Google Scholar] [CrossRef]
- Nygren, J.; Thorell, A.; Ljungqvist, O. Preoperative oral carbohydrate therapy. Curr. Opin. Anaesthesiol. 2015, 28, 364–369. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ticha, M.; Sobotka, O.; Skorepa, P.; Sobotka, L. Beneficial Role of Increased Glucose Infusion in Decompensated Type 2 Diabetes Patient. Diabetology 2025, 6, 47. https://doi.org/10.3390/diabetology6060047
Ticha M, Sobotka O, Skorepa P, Sobotka L. Beneficial Role of Increased Glucose Infusion in Decompensated Type 2 Diabetes Patient. Diabetology. 2025; 6(6):47. https://doi.org/10.3390/diabetology6060047
Chicago/Turabian StyleTicha, Marie, Ondrej Sobotka, Pavel Skorepa, and Lubos Sobotka. 2025. "Beneficial Role of Increased Glucose Infusion in Decompensated Type 2 Diabetes Patient" Diabetology 6, no. 6: 47. https://doi.org/10.3390/diabetology6060047
APA StyleTicha, M., Sobotka, O., Skorepa, P., & Sobotka, L. (2025). Beneficial Role of Increased Glucose Infusion in Decompensated Type 2 Diabetes Patient. Diabetology, 6(6), 47. https://doi.org/10.3390/diabetology6060047




