Sodium-Glucose Co-Transporter 2 Inhibitors as a Powerful Cardioprotective and Renoprotective Tool: Overview of Clinical Trials and Mechanisms
Abstract
1. Introduction
2. Cardiovascular and Renal Events in the Setting of Type 2 Diabetes Mellitus Clinical Trials
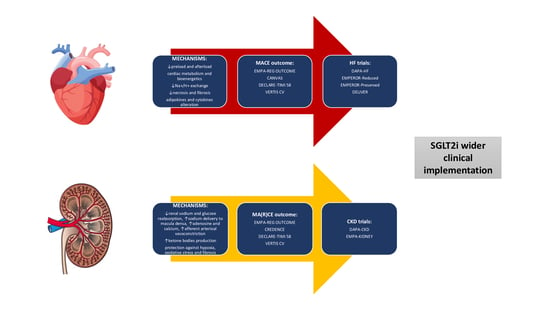
3. Cardioprotective Effects and Mechanisms of SGLT2 Inhibitors
4. Renoprotective Effects and Mechanisms of SGLT2 Inhibitors
5. Brief Discussion on Existing Challenges and Gaps in the Research and Application of SGLT2 Inhibitors
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CI | confidence interval |
| CKD | chronic kidney disease |
| CVOT | cardiovascular outcomes trial |
| GFR | glomerular filtration |
| HbA1c | glycated hemoglobin |
| HF | heart failure |
| HR | hazard ratio |
| mrEF | mildly reduced ejection fraction |
| NYHA | New York Hear Association |
| pEF | preserved ejection fraction |
| rEF | reduced ejection fraction |
| SGLT2i | Sodium-glucose co-transporter 2 inhibitors |
References
- Rahelić, D.; Altabas, V.; Bakula, M.; Balić, S.; Balint, I.; Marković, B.B.; Bicanić, N.; Bjelinski, I.; Bozikov, V.; Varzić, S.C.; et al. Croatian guidelines for the pharmacotherapy of type 2 diabetes. Lijec. Vjesn. 2016, 138, 1–21. [Google Scholar]
- European Medicines Agency. Available online: https://www.ema.europa.eu/en/medicines (accessed on 12 June 2023).
- Scheen, A.J. Pharmacodynamics, efficacy and safety of sodium-glucose co-transporter type 2 (SGLT2) inhibitors for the treatment of type 2 diabetes mellitus. Drugs 2015, 75, 33–59. [Google Scholar] [CrossRef]
- Wright, E.M. SGLT2 Inhibitors: Physiology and Pharmacology. Kidney360 2021, 17, 2027–2037. [Google Scholar] [CrossRef]
- Donnan, J.R.; Grandy, C.A.; Chibrikov, E.; Marra, C.A.; Aubrey-Bassler, K.; Johnston, K.; Swab, M.; Hache, J.; Curnew, D.; Nguyen, H.; et al. Comparative safety of the sodium glucose co-transporter 2 (SGLT2) inhibitors: A systematic review and meta-analysis. BMJ Open 2019, 9, e022577. [Google Scholar] [CrossRef] [PubMed]
- Musso, G.; Gambino, R.; Cassader, M.; Pagano, G. A novel approach to control hyperglycemia in type 2 diabetes: Sodium glucose co-transport (SGLT) inhibitors: Systematic review and meta-analysis of randomized trials. Ann. Med. 2012, 44, 375–393. [Google Scholar] [CrossRef]
- Clar, C.; Gill, J.A.; Court, R.; Waugh, N. Systematic review of SGLT2 receptor inhibitors in dual or triple therapy in type 2 diabetes. BMJ Open 2012, 2, e001007. [Google Scholar] [CrossRef]
- Vasilakou, D.; Karagiannis, T.; Athanasiadou, E.; Mainou, M.; Liakos, A.; Bekiari, E.; Sarigianni, M.; Matthews, D.R.; Tsapas, A. Sodium-glucose cotransporter 2 inhibitors for type 2 diabetes: A systematic review and meta-analysis. Ann. Intern. Med. 2013, 159, 262–274. [Google Scholar] [CrossRef] [PubMed]
- Berhan, A.; Barker, A. Sodium glucose co-transport 2 inhibitors in the treatment of type 2 diabetes mellitus: A meta-analysis of randomized double-blind controlled trials. BMC Endocr. Disord. 2013, 13, 58. [Google Scholar] [CrossRef]
- Lo, C.W.H.; Fei, Y.; Cheung, B.M.Y. Cardiovascular Outcomes in Trials of New Antidiabetic Drug Classes. Card. Fail. Rev. 2021, 7, e04. [Google Scholar] [CrossRef]
- Mazin, I.; Chernomordik, F.; Fefer, P.; Matetzky, S.; Beigel, R. The Impact of Novel Anti-Diabetic Medications on CV Outcomes: A New Therapeutic Horizon for Diabetic and Non-Diabetic Cardiac Patients. J. Clin. Med. 2022, 11, 1904. [Google Scholar] [CrossRef] [PubMed]
- Rangaswami, J.; Bhalla, V.; de Boer, I.H.; Staruschenko, A.; Sharp, J.A.; Singh, R.R.; Lo, K.B.; Tuttle, K.; Vaduganathan, M.; Ventura, H.; et al. Cardiorenal Protection With the Newer Antidiabetic Agents in Patients With Diabetes and Chronic Kidney Disease: A Scientific Statement From the American Heart Association. Circulation 2020, 142, e265–e286. [Google Scholar] [CrossRef]
- Ferro, E.G.; Elshazly, M.B.; Bhatt, D.L. New Antidiabetes Medications and Their Cardiovascular and Renal Benefits. Cardiol. Clin. 2021, 39, 335–351. [Google Scholar] [CrossRef]
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Woerle, H.J.; et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N. Engl. J. Med. 2015, 373, 2117–2128. [Google Scholar] [CrossRef]
- Khokhlov, A.; Vorobjev, S.; Mirolyubova, O.; Boldueva, S.; Ershova, O.; Ballyzek, M.; Smolenskaya, O.; Yakushin, S.S.; Zateyshchikov, D.; Arkhipov, M.; et al. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N. Engl. J. Med. 2017, 377, 644–657. [Google Scholar] [CrossRef]
- Perkovic, V.; Jardine, M.J.; Neal, B.; Bompoint, S.; Heerspink, H.J.L.; Charytan, D.M.; Edwards, R.; Agarwal, R.; Bakris, G.; Bull, S.; et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N. Engl. J. Med. 2019, 380, 2295–2306. [Google Scholar] [CrossRef] [PubMed]
- Wiviott, S.D.; Raz, I.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; Silverman, M.G.; Zelniker, T.A.; Kuder, J.F.; Murphy, S.A.; et al. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2019, 380, 347–357. [Google Scholar] [CrossRef]
- Cannon, C.P.; Pratley, R.; Dagogo-Jack, S.; Mancuso, J.; Huyck, S.; Masiukiewicz, U.; Charbonnel, B.; Frederich, R.; Gallo, S.; Cosentino, F.; et al. Cardiovascular Outcomes with Ertugliflozin in Type 2 Diabetes. N. Engl. J. Med. 2020, 383, 1425–1435. [Google Scholar] [CrossRef]
- Verma, S.; McMurray, J.J.V. SGLT2 inhibitors and mechanisms of cardiovascular benefit: A state-of-the-art review. Diabetologia 2018, 61, 2108–2117. [Google Scholar] [CrossRef] [PubMed]
- Lopaschuk, G.D.; Verma, S. Mechanisms of Cardiovascular Benefits of Sodium Glucose Co-Transporter 2 (SGLT2) Inhibitors: A State-of-the-Art Review. JACC Basic Transl. Sci. 2020, 5, 632–644. [Google Scholar] [CrossRef]
- Salvatore, T.; Galiero, R.; Caturano, A.; Rinaldi, L.; Di Martino, A.; Albanese, G.; Di Salvo, J.; Epifani, R.; Marfella, R.; Docimo, G.; et al. An Overview of the Cardiorenal Protective Mechanisms of SGLT2 Inhibitors. Int. J. Mol. Sci. 2022, 23, 3651. [Google Scholar] [CrossRef]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef] [PubMed]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Pocock, S.J.; Carson, P.; Januzzi, J.; Verma, S.; Tsutsui, H.; Brueckmann, M.; et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N. Engl. J. Med. 2020, 383, 1413–1424. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2022, 24, 4–131. [Google Scholar] [CrossRef]
- Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Bocchi, E.; Böhm, M.; Brunner-La Rocca, H.P.; Choi, D.J.; Chopra, V.; Chuquiure-Valenzuela, E.; et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N. Engl. J. Med. 2021, 385, 1451–1461. [Google Scholar] [CrossRef]
- Solomon, S.D.; McMurray, J.J.V.; Claggett, B.; de Boer, R.A.; DeMets, D.; Hernandez, A.F.; Inzucchi, S.E.; Kosiborod, M.N.; Lam, C.S.P.; Martinez, F.; et al. Dapagliflozin in Heart Failure with Mildly Reduced or Preserved Ejection Fraction. N. Engl. J. Med. 2022, 387, 1089–1098. [Google Scholar] [CrossRef]
- Vaduganathan, M.; Docherty, K.F.; Claggett, B.L.; Jhund, P.S.; de Boer, R.A.; Hernandez, A.F.; Inzucchi, S.E.; Kosiborod, M.N.; Lam, C.S.P.; Martinez, F.; et al. SGLT-2 inhibitors in patients with heart failure: A comprehensive meta-analysis of five randomised controlled trials. Lancet 2022, 400, 757–767. [Google Scholar] [CrossRef]
- Ravindran, S.; Munusamy, S. Renoprotective mechanisms of sodium-glucose co-transporter 2 (SGLT2) inhibitors against the progression of diabetic kidney disease. J. Cell Physiol. 2022, 237, 1182–1205. [Google Scholar] [CrossRef]
- Skrabic, R.; Kumric, M.; Vrdoljak, J.; Rusic, D.; Skrabic, I.; Vilovic, M.; Martinovic, D.; Duplancic, V.; Ticinovic Kurir, T.; Bozic, J. SGLT2 Inhibitors in Chronic Kidney Disease: From Mechanisms to Clinical Practice. Biomedicines 2022, 10, 2458. [Google Scholar] [CrossRef] [PubMed]
- Heerspink, H.J.L.; Stefánsson, B.V.; Correa-Rotter, R.; Chertow, G.M.; Greene, T.; Hou, F.F.; Mann, J.F.E.; McMurray, J.J.V.; Lindberg, M.; Rossing, P.; et al. Dapagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2020, 383, 1436–1446. [Google Scholar] [CrossRef]
- Herrington, W.G.; Staplin, N.; Wanner, C.; Green, J.B.; Hauske, S.J.; Emberson, J.R.; Preiss, D.; Judge, P.; Mayne, K.J.; Ng, S.Y.A.; et al. Empagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2023, 388, 117–127. [Google Scholar] [CrossRef]
- Nuffield Department of Population Health Renal Studies Group; SGLT2 inhibitor Meta-Analysis Cardio-Renal Trialists’ Consortium. Impact of diabetes on the effects of sodium glucose co-transporter-2 inhibitors on kidney outcomes: Collaborative meta-analysis of large placebo-controlled trials. Lancet 2022, 400, 1788–1801. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, R.; Jha, K.; Dardari, Z.; Heyward, J.; Blumenthal, R.S.; Eckel, R.H.; Alexander, G.C.; Blaha, M.J. National Trends in Use of Sodium-Glucose Cotransporter-2 Inhibitors and Glucagon-like Peptide-1 Receptor Agonists by Cardiologists and Other Specialties, 2015 to 2020. J. Am. Heart Assoc. 2022, 11, e023811. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, A.; Shankar, M.; Lerma, E.V.; Wiegley, N.; GlomCon Editorial Team. Sodium Glucose Cotransporter 2 (SGLT2) Inhibitors and CKD: Are You a #Flozinator? Kidney Med. 2023, 5, 100608. [Google Scholar] [CrossRef] [PubMed]
- Schernthaner, G.; Shehadeh, N.; Ametov, A.S.; Bazarova, A.V.; Ebrahimi, F.; Fasching, P.; Janež, A.; Kempler, P.; Konrāde, I.; Lalić, N.M.; et al. Worldwide inertia to the use of cardiorenal protective glucose-lowering drugs (SGLT2i and GLP-1 RA) in high-risk patients with type 2 diabetes. Cardiovasc. Diabetol. 2020, 19, 185. [Google Scholar] [CrossRef]
- Selwyn, J.; Pichardo-Lowden, A.R. Managing Hospitalized Patients Taking SGLT2 Inhibitors: Reducing the Risk of Euglycemic Diabetic Ketoacidosis. Diabetology 2023, 4, 86–92. [Google Scholar] [CrossRef]
- Fadini, G.P.; Del Prato, S.; Avogaro, A.; Solini, A. Challenges and opportunities in real-world evidence on the renal effects of sodium-glucose cotransporter-2 inhibitors. Diabetes Obes. Metab. 2022, 24, 177–186. [Google Scholar] [CrossRef]
- Bellary, S.; Barnett, A.H. SGLT2 inhibitors in older adults: Overcoming the age barrier. Lancet Healthy Longev. 2023, 4, e127–e128. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belančić, A.; Klobučar, S. Sodium-Glucose Co-Transporter 2 Inhibitors as a Powerful Cardioprotective and Renoprotective Tool: Overview of Clinical Trials and Mechanisms. Diabetology 2023, 4, 251-258. https://doi.org/10.3390/diabetology4030022
Belančić A, Klobučar S. Sodium-Glucose Co-Transporter 2 Inhibitors as a Powerful Cardioprotective and Renoprotective Tool: Overview of Clinical Trials and Mechanisms. Diabetology. 2023; 4(3):251-258. https://doi.org/10.3390/diabetology4030022
Chicago/Turabian StyleBelančić, Andrej, and Sanja Klobučar. 2023. "Sodium-Glucose Co-Transporter 2 Inhibitors as a Powerful Cardioprotective and Renoprotective Tool: Overview of Clinical Trials and Mechanisms" Diabetology 4, no. 3: 251-258. https://doi.org/10.3390/diabetology4030022
APA StyleBelančić, A., & Klobučar, S. (2023). Sodium-Glucose Co-Transporter 2 Inhibitors as a Powerful Cardioprotective and Renoprotective Tool: Overview of Clinical Trials and Mechanisms. Diabetology, 4(3), 251-258. https://doi.org/10.3390/diabetology4030022