Gut Metabolism of Sugars: Formation of Glycotoxins and Their Intestinal Absorption
Abstract
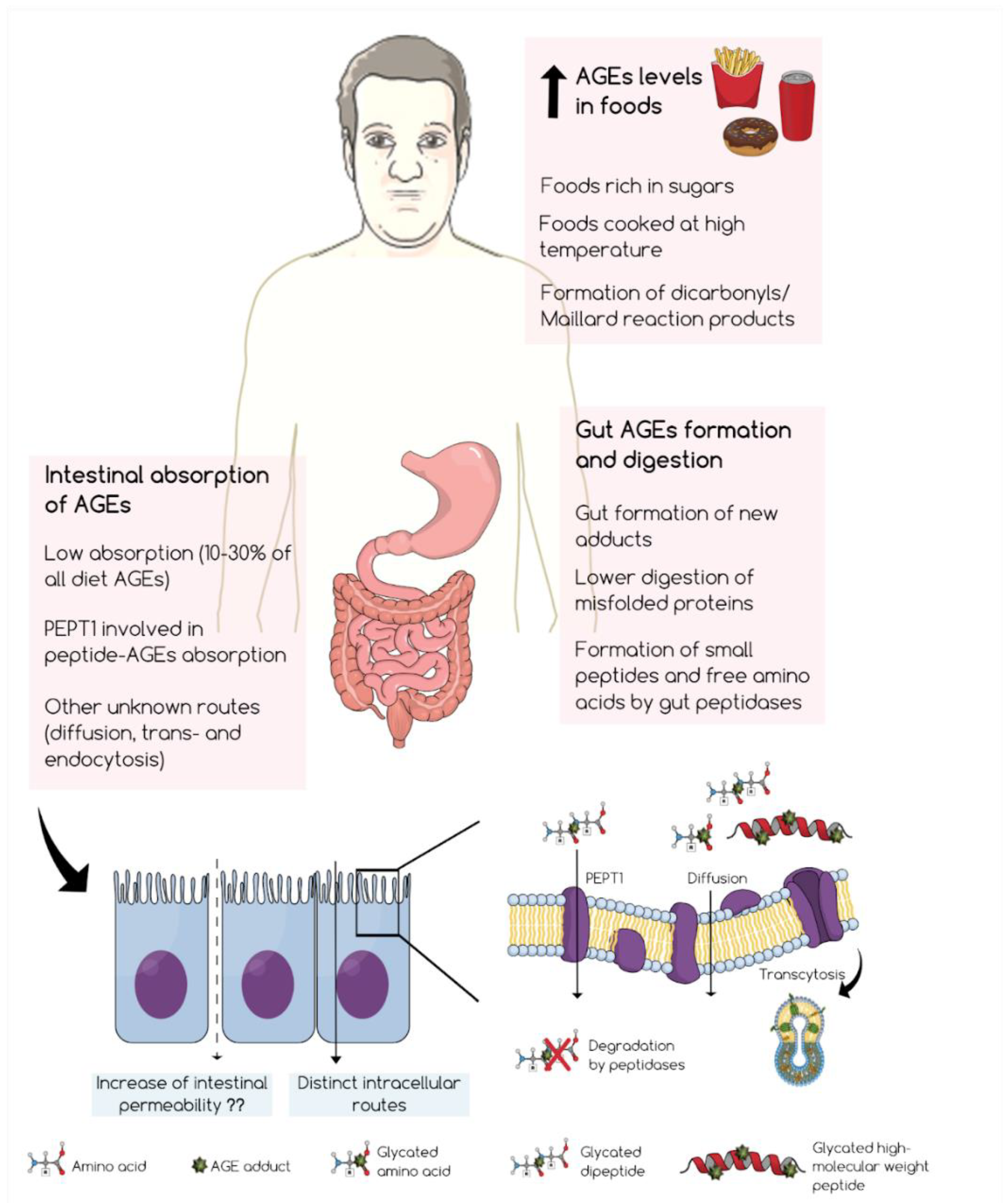
1. Introduction
2. Formation of Glycotoxins in the Gut and Their Digestion
3. Intestinal Absorption of Glycotoxins
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AGEs | advanced glycation end-products |
| CEL | (carboxyethyl)lysine |
| CMA | pg 8 só |
| CML | NE-(carboxymethyl)-lysine |
| G-H1 | hydroimidazolone 1 derived from glyoxal |
| GLO | glyoxalase system |
| GO | glyoxal |
| MG | methylglyoxal |
| MG-H1 | Nδ-(5-hydro-5-methyl-4-imidazolon-2-yl)-ornithine |
| MOLD | methylglyoxal lysine dimer |
| PEPT1 | peptide transporter |
References
- Matafome, P.; Rodrigues, T.; Sena, C.; Seiça, R. Methylglyoxal in Metabolic Disorders: Facts, Myths, and Promises. Med. Res. Rev. 2017, 37, 368–403. [Google Scholar] [CrossRef] [PubMed]
- Allaman, I.; Bélanger, M.; Magistretti, P.J. Methylglyoxal, the dark side of glycolysis. Front. Neurosci. 2015, 9, 23. [Google Scholar] [CrossRef] [PubMed]
- Kalapos, M.P. Where does plasma methylglyoxal originate from? Diabetes Res. Clin. Pract. 2013, 99, 260–271. [Google Scholar] [CrossRef]
- Poulsen, M.W.; Hedegaard, R.V.; Andersen, J.M.; de Courten, B.; Bügel, S.; Nielsen, J.; Skibsted, L.H.; Dragsted, L.O. Advanced glycation endproducts in food and their effects on health. Food Chem. Toxicol. 2013, 60, 10–37. [Google Scholar] [CrossRef] [PubMed]
- Baynes, J.W.; Thorpe, S.R. Glycoxidation and lipoxidation in atherogenesis. Free Radic. Biol. Med. 2000, 28, 1708–1716. [Google Scholar] [CrossRef]
- Kumagai, T.; Nangaku, M.; Kojima, I.; Nagai, R.; Ingelfinger, J.R.; Miyata, T.; Fujita, T.; Inagi, R. Glyoxalase I overexpression ameliorates renal ischemia-reperfusion injury in rats. Am. J. Physiol. Renal Physiol. 2009, 296, F912–F921. [Google Scholar] [CrossRef]
- Roberts, M.J.; Wondrak, G.T.; Laurean, D.C.; Jacobson, M.K.; Jacobson, E.L. DNA damage by carbonyl stress in human skin cells. Mutat. Res. Mol. Mech. Mutagen. 2003, 522, 45–56. [Google Scholar] [CrossRef]
- Schalkwijk, C.; Stehouwer, C.D.A. Methylglyoxal, a Highly Reactive Dicarbonyl Compound, in Diabetes, Its Vascular Complications, and Other Age-Related Diseases. Physiol. Rev. 2020, 100, 407–461. [Google Scholar] [CrossRef]
- Liang, Z.; Chen, X.; Li, L.; Li, B.; Yang, Z. The fate of dietary advanced glycation end products in the body: From oral intake to excretion. Crit. Rev. Food Sci. Nutr. 2020, 60, 3475–3491. [Google Scholar] [CrossRef]
- Matafome, P. Another Player in the Field: Involvement of Glycotoxins and Glycosative Stress in Insulin Secretion and Resistance. Diabetology 2020, 1, 24–36. [Google Scholar] [CrossRef]
- Frolova, N.; Soboleva, A.; Nguyen, V.D.; Kim, A.; Ihling, C.; Eisenschmidt-Bönn, D.; Mamontova, T.; Herfurth, U.M.; Wessjohann, L.A.; Sinz, A.; et al. Probing glycation potential of dietary sugars in human blood by an integrated in vitro approach. Food Chem. 2021, 347, 128951. [Google Scholar] [CrossRef] [PubMed]
- Almajwal, A.M.; Alam, I.; Abulmeaty, M.; Razak, S.; Pawelec, G.; Alam, W. Intake of dietary advanced glycation end products influences inflammatory markers, immune phenotypes, and antiradical capacity of healthy elderly in a little-studied population. Food Sci. Nutr. 2020, 8, 1046–1057. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Andrade, C.; Tessier, F.J.; Niquet-Leridon, C.; Seiquer, I.; Navarro, M.P. Study of the urinary and faecal excretion of N ε-carboxymethyllysine in young human volunteers. Amino Acids 2012, 43, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Nie, C.; Li, Y.; Qian, H.; Ying, H.; Wang, L. Advanced glycation end products in food and their effects on intestinal tract. Crit. Rev. Food Sci. Nutr. 2022, 62, 3103–3115. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Andrade, C.; Fogliano, V. Dietary Advanced Glycosylation End-Products (dAGEs) and Melanoidins Formed through the Maillard Reaction: Physiological Consequences of their Intake. Annu. Rev. Food Sci. Technol. 2018, 9, 271–291. [Google Scholar] [CrossRef] [PubMed]
- Henle, T. AGEs in foods: Do they play a role in uremia? Kidney Int. 2003, 63, S145–S147. [Google Scholar] [CrossRef]
- Henle, T. Protein-bound advanced glycation endproducts (AGEs) as bioactive amino acid derivatives in foods. Amino Acids 2005, 29, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.; Sun, X.; Zhang, Y.; Yang, T.; Wang, C.; Zhang, J.; Duan, Z.; Shang, F.; Fan, J.; Liu, Y.; et al. Effect of pectin oligosaccharides supplementation on infant formulas: The storage stability, formation and intestinal absorption of advanced glycation end products. Food Chem. 2022, 373, 131571. [Google Scholar] [CrossRef]
- Bains, Y.; Gugliucci, A.; Caccavello, R. Advanced glycation endproducts form during ovalbumin digestion in the presence of fructose: Inhibition by chlorogenic acid. Fitoterapia 2017, 120, 1–5. [Google Scholar] [CrossRef]
- Monteiro-Alfredo, T.; Oliveira, S.; Amaro, A.; Rosendo-Silva, D.; Antunes, K.; Pires, A.S.; Teixo, R.; Abrantes, A.M.; Botelho, M.F.; Castelo-Branco, M.; et al. Hypoglycaemic and Antioxidant Properties of Acrocomia aculeata (Jacq.) Lodd Ex Mart. Extract Are Associated with Better Vascular Function of Type 2 Diabetic Rats. Nutrients 2021, 13, 2856. [Google Scholar] [CrossRef]
- Monteiro-Alfredo, T.; Caramelo, B.; Arbeláez, D.; Amaro, A.; Barra, C.; Silva, D.; Oliveira, S.; Seiça, R.; Matafome, P. Distinct Impact of Natural Sugars from Fruit Juices and Added Sugars on Caloric Intake, Body Weight, Glycaemia, Oxidative Stress and Glycation in Diabetic Rats. Nutrients 2021, 13, 2956. [Google Scholar] [CrossRef] [PubMed]
- Slavin, J.; Carlson, J. Carbohydrates. Adv. Nutr. 2014, 5, 760–761. [Google Scholar] [CrossRef] [PubMed]
- Uribarri, J.; Cai, W.; Woodward, M.; Tripp, E.; Goldberg, L.; Pyzik, R.; Yee, K.; Tansman, L.; Chen, X.; Mani, V.; et al. Elevated Serum Advanced Glycation Endproducts in Obese Indicate Risk for the Metabolic Syndrome: A Link Between Healthy and Unhealthy Obesity? J. Clin. Endocrinol. Metab. 2015, 100, 1957–1966. [Google Scholar] [CrossRef]
- Ryan-Harshman, M.; Aldoori, W. New Dietary Reference Intakes for Macronutrients and Fibre. Can Fam Physician 2006, 52, 177–179. [Google Scholar]
- CDC. Know Your Limit for Added Sugars. Available online: https://www.cdc.gov/nutrition/data-statistics/added-sugars.html (accessed on 21 September 2022).
- Dashty, M. A quick look at biochemistry: Carbohydrate metabolism. Clin. Biochem. 2013, 46, 1339–1352. [Google Scholar] [CrossRef] [PubMed]
- Erichsen, J.M.; Fadel, J.R.; Reagan, L.P. Peripheral versus central insulin and leptin resistance: Role in metabolic disorders, cognition, and neuropsychiatric diseases. Neuropharmacology 2022, 203, 108877. [Google Scholar] [CrossRef]
- Tappy, L. Metabolism of sugars: A window to the regulation of glucose and lipid homeostasis by splanchnic organs. Clin. Nutr. 2021, 40, 1691–1698. [Google Scholar] [CrossRef] [PubMed]
- Nagai, R.; Shirakawa, J.-I.; Fujiwara, Y.; Ohno, R.-I.; Moroishi, N.; Sakata, N.; Nagai, M. Detection of AGEs as markers for carbohydrate metabolism and protein denaturation. J. Clin. Biochem. Nutr. 2014, 55, 1–6. [Google Scholar] [CrossRef]
- Hellwig, M.; Gensberger-Reigl, S.; Henle, T.; Pischetsrieder, M. Food-derived 1,2-dicarbonyl compounds and their role in diseases. Semin. Cancer Biol. 2018, 49, 1–8. [Google Scholar] [CrossRef]
- Degen, J.; Vogel, M.; Richter, D.; Hellwig, M.; Henle, T. Metabolic Transit of Dietary Methylglyoxal. J. Agric. Food Chem. 2013, 61, 10253–10260. [Google Scholar] [CrossRef]
- Papetti, A.; Mascherpa, D.; Gazzani, G. Free α-dicarbonyl compounds in coffee, barley coffee and soy sauce and effects of in vitro digestion. Food Chem. 2014, 164, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Li, L.; Le, T.T.; Larsen, L.B.; Su, G.; Liang, Y.; Li, B. Digestibility of Glyoxal-Glycated β-Casein and β-Lactoglobulin and Distribution of Peptide-Bound Advanced Glycation End Products in Gastrointestinal Digests. J. Agric. Food Chem. 2017, 65, 5778–5788. [Google Scholar] [CrossRef] [PubMed]
- Moscovici, A.M.; Joubran, Y.; Briard-Bion, V.; Mackie, A.; Dupont, D.; Lesmes, U. The impact of the Maillard reaction on the in vitro proteolytic breakdown of bovine lactoferrin in adults and infants. Food Funct. 2014, 5, 1898–1908. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Sheng, B.; Wu, Y.; Li, H.; Xu, D.; Nian, Y.; Mao, S.; Li, C.; Xu, X.; Zhou, G. Comparison of Free and Bound Advanced Glycation End Products in Food: A Review on the Possible Influence on Human Health. J. Agric. Food Chem. 2019, 67, 14007–14018. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Saez, N.; Fernandez-Gomez, B.; Cai, W.; Uribarri, J.; del Castillo, M.D. In vitro formation of Maillard reaction products during simulated digestion of meal-resembling systems. Food Res. Int. 2019, 118, 72–80. [Google Scholar] [CrossRef]
- van der Lugt, T.; Venema, K.; van Leeuwen, S.; Vrolijk, M.F.; Opperhuizen, A.; Bast, A. Gastrointestinal digestion of dietary advanced glycation endproducts using anin vitromodel of the gastrointestinal tract (TIM-1). Food Funct. 2020, 11, 6297–6307. [Google Scholar] [CrossRef]
- Hellwig, M.; Matthes, R.; Peto, A.; Löbner, J.; Henle, T. N-ε-fructosyllysine and N-ε-carboxymethyllysine, but not lysinoalanine, are available for absorption after simulated gastrointestinal digestion. Amino Acids 2014, 46, 289–299. [Google Scholar] [CrossRef]
- Heianza, Y.; Sun, D.; Li, X.; DiDonato, J.A.; Bray, G.A.; Sacks, F.M.; Qi, L. Gut microbiota metabolites, amino acid metabolites and improvements in insulin sensitivity and glucose metabolism: The POUNDS Lost trial. Gut 2019, 68, 263–270. [Google Scholar] [CrossRef]
- Kashani, A.; Brejnrod, A.D.; Jin, C.; Kern, T.; Madsen, A.N.; Holm, L.A.; Gerber, G.K.; Holm, J.-C.; Hansen, T.; Holst, B.; et al. Impaired glucose metabolism and altered gut microbiome despite calorie restriction of ob/ob mice. Anim. Microbiome 2019, 1, 11. [Google Scholar] [CrossRef]
- Kawano, Y.; Edwards, M.; Huang, Y.; Bilate, A.M.; Araujo, L.P.; Tanoue, T.; Atarashi, K.; Ladinsky, M.S.; Reiner, S.L.; Wang, H.H.; et al. Microbiota imbalance induced by dietary sugar disrupts immune-mediated protection from metabolic syndrome. Cell 2022, 185, 3501–3519.e20. [Google Scholar] [CrossRef]
- Morales, F.J.; Somoza, V.; Fogliano, V. Physiological relevance of dietary melanoidins. Amino Acids 2012, 42, 1097–1109. [Google Scholar] [CrossRef] [PubMed]
- Mastrocola, R.; Collotta, D.; Gaudioso, G.; Le Berre, M.; Cento, A.; Ferreira, G.A.; Chiazza, F.; Verta, R.; Bertocchi, I.; Manig, F.; et al. Effects of Exogenous Dietary Advanced Glycation End Products on the Cross-Talk Mechanisms Linking Microbiota to Metabolic Inflammation. Nutrients 2020, 12, 2497. [Google Scholar] [CrossRef]
- Wang, J.; Cai, W.; Yu, J.; Liu, H.; He, S.; Zhu, L.; Xu, J. Dietary Advanced Glycation End Products Shift the Gut Microbiota Composition and Induce Insulin Resistance in Mice. Diabetes Metab. Syndr. Obes. 2022, 15, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Amoroso, A.; Maga, G.; Daglia, M. Cytotoxicity of α-dicarbonyl compounds submitted to in vitro simulated digestion process. Food Chem. 2013, 140, 654–659. [Google Scholar] [CrossRef]
- Maessen, D.E.; Hanssen, N.M.; Scheijen, J.L.; van der Kallen, C.J.; van Greevenbroek, M.M.; Stehouwer, C.D.; Schalkwijk, C.G. Post–Glucose Load Plasma α-Dicarbonyl Concentrations Are Increased in Individuals with Impaired Glucose Metabolism and Type 2 Diabetes: The CODAM Study. Diabetes Care 2015, 38, 913–920. [Google Scholar] [CrossRef] [PubMed]
- Palaseweenun, P.; Hagen-Plantinga, E.A.; Schonewille, J.T.; Koop, G.; Butre, C.; Jonathan, M.; Wierenga, P.A.; Hendriks, W.H. Urinary excretion of advanced glycation end products in dogs and cats. J. Anim. Physiol. Anim. Nutr. 2021, 105, 149–156. [Google Scholar] [CrossRef]
- Scheijen, J.L.; Hanssen, N.M.; Van Greevenbroek, M.M.; Van Der Kallen, C.J.; Feskens, E.J.; Stehouwer, C.D.; Schalkwijk, C.G. Dietary intake of advanced glycation endproducts is associated with higher levels of advanced glycation endproducts in plasma and urine: The CODAM study. Clin. Nutr. 2018, 37, 919–925. [Google Scholar] [CrossRef]
- Forster, S.; Gariballa, S. Age as a determinant of nutritional status: A cross sectional study. Nutr. J. 2005, 4, 28. [Google Scholar] [CrossRef]
- Hellwig, M.; Geissler, S.; Matthes, R.; Peto, A.; Silow, C.; Brandsch, M.; Henle, T. Transport of Free and Peptide-Bound Glycated Amino Acids: Synthesis, Transepithelial Flux at Caco-2 Cell Monolayers, and Interaction with Apical Membrane Transport Proteins. ChemBioChem 2011, 12, 1270–1279. [Google Scholar] [CrossRef]
- Hellwig, M.; Geissler, S.; Peto, A.; Knütter, I.; Brandsch, M.; Henle, T. Transport of Free and Peptide-Bound Pyrraline at Intestinal and Renal Epithelial Cells. J. Agric. Food Chem. 2009, 57, 6474–6480. [Google Scholar] [CrossRef]
- Grunwald, S.; Krause, R.; Bruch, M.; Henle, T.; Brandsch, M. Transepithelial flux of early and advanced glycation compounds across Caco-2 cell monolayers and their interaction with intestinal amino acid and peptide transport systems. Br. J. Nutr. 2006, 95, 1221–1228. [Google Scholar] [CrossRef] [PubMed]
- Dubois, C.; Litke, R.; Rianha, S.; Paul-Constant, C.; Guidice, J.-M.L.; Taront, S.; Tessier, F.J.; Boulanger, E.; Fradin, C. Exposure of Caenorhabditis elegans to Dietary Nε-Carboxymethyllysine Emphasizes Endocytosis as a New Route for Intestinal Absorption of Advanced Glycation End Products. Nutrients 2021, 13, 4398. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Chen, Y.; Ouyang, Y.; He, Y.; Xiao, J.; Zhang, L.; Feng, N. Effect of catechin on dietary AGEs absorption and cytotoxicity in Caco-2 cells. Food Chem. 2021, 355, 129574. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-Y.; Lee, H.-B.; Lee, S.-Y.; Oh, M.-J.; Ha, S.K.; Do, E.; Lee, H.H.L.; Hur, J.; Lee, K.-W.; Nam, M.-H.; et al. Lactococcus lactis KF140 Reduces Dietary Absorption of Nε—(Carboxymethyl)lysine in Rats and Humans via β-Galactosidase Activity. Front. Nutr. 2022, 9, 916262. [Google Scholar] [CrossRef] [PubMed]
- Rigoldi, F.; Spero, L.; Vedove, A.D.; Redaelli, A.; Parisini, E.; Gautieri, A. Molecular dynamics simulations provide insights into the substrate specificity of FAOX family members. Mol. BioSyst. 2016, 12, 2622–2633. [Google Scholar] [CrossRef]
- Chegão, A.; Guarda, M.; Alexandre, B.M.; Shvachiy, L.; Temido-Ferreira, M.; Marques-Morgado, I.; Gomes, B.F.; Matthiesen, R.; Lopes, L.V.; Florindo, P.R.; et al. Glycation modulates glutamatergic signaling and exacerbates Parkinson’s disease-like phenotypes. NPJ Park. Dis. 2022, 8, 51. [Google Scholar] [CrossRef]
- He, C.; Sabol, J.; Mitsuhashi, T.; Vlassara, H. Dietary glycotoxins: Inhibition of reactive products by aminoguanidine facilitates renal clearance and reduces tissue sequestration. Diabetes 1999, 48, 1308–1315. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monteiro-Alfredo, T.; Matafome, P. Gut Metabolism of Sugars: Formation of Glycotoxins and Their Intestinal Absorption. Diabetology 2022, 3, 596-605. https://doi.org/10.3390/diabetology3040045
Monteiro-Alfredo T, Matafome P. Gut Metabolism of Sugars: Formation of Glycotoxins and Their Intestinal Absorption. Diabetology. 2022; 3(4):596-605. https://doi.org/10.3390/diabetology3040045
Chicago/Turabian StyleMonteiro-Alfredo, Tamaeh, and Paulo Matafome. 2022. "Gut Metabolism of Sugars: Formation of Glycotoxins and Their Intestinal Absorption" Diabetology 3, no. 4: 596-605. https://doi.org/10.3390/diabetology3040045
APA StyleMonteiro-Alfredo, T., & Matafome, P. (2022). Gut Metabolism of Sugars: Formation of Glycotoxins and Their Intestinal Absorption. Diabetology, 3(4), 596-605. https://doi.org/10.3390/diabetology3040045






